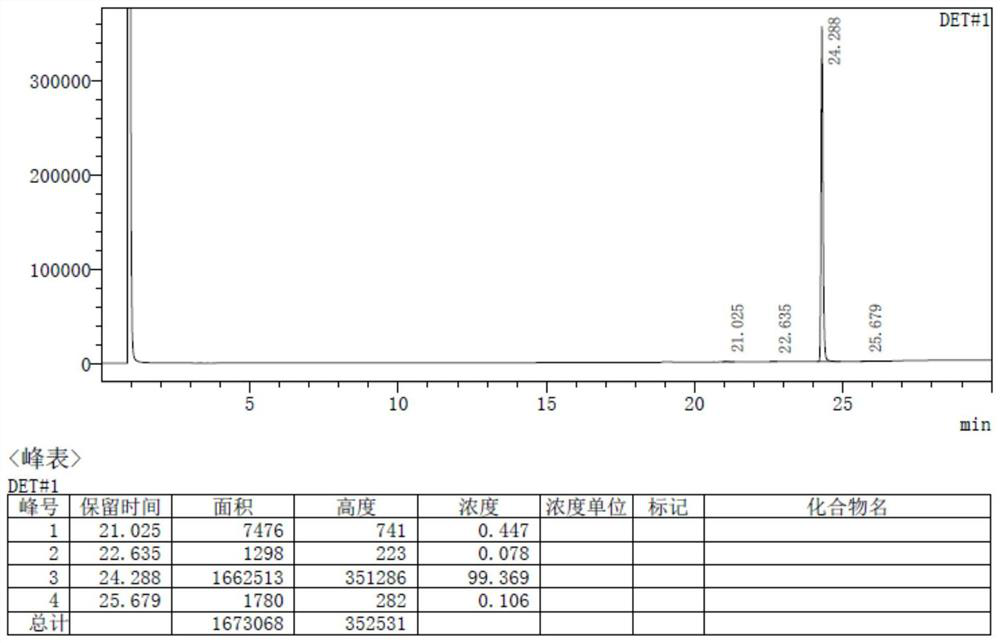
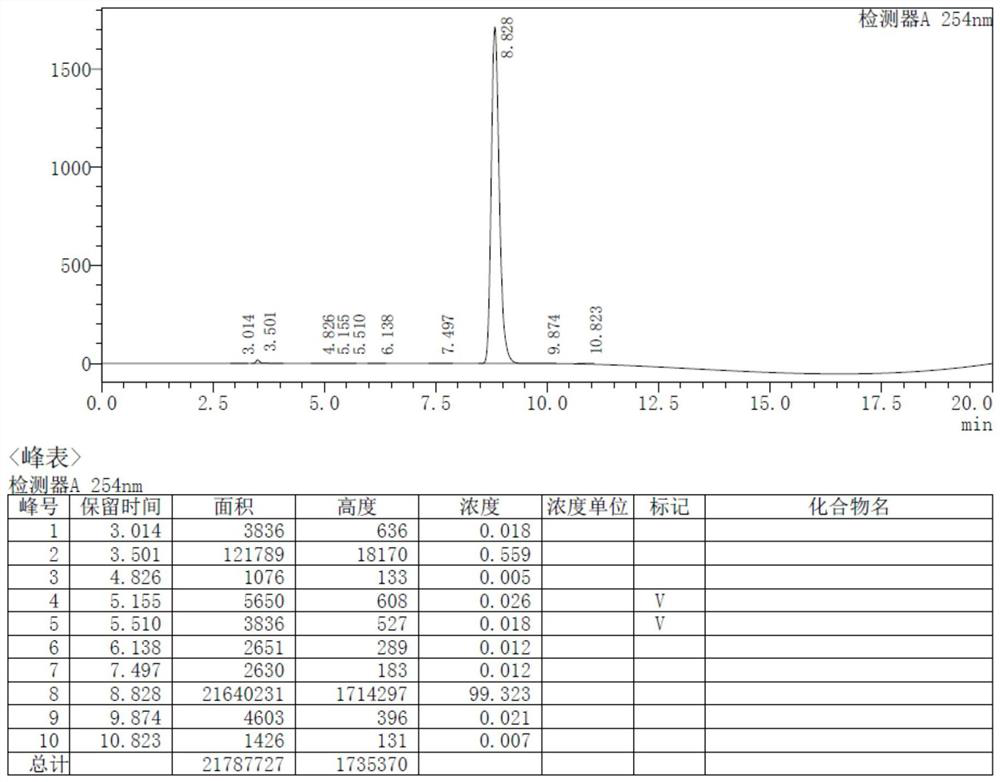
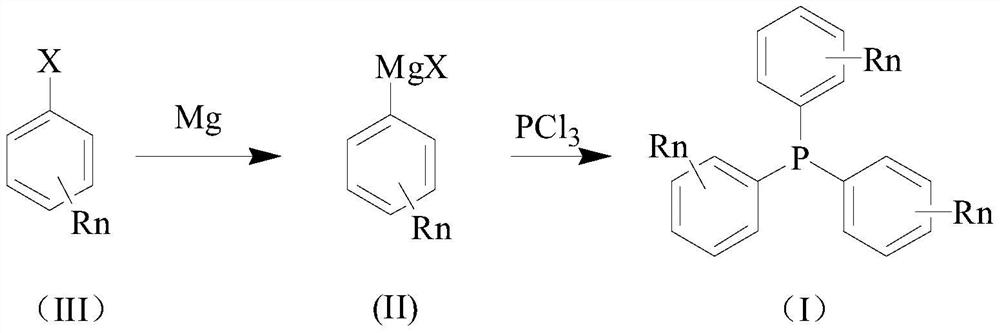
Synthesis method of triphenylphosphine derivative tri-(R-phenyl) phosphine
A synthetic method and phenyl technology, applied in the field of organic synthesis, can solve problems such as unsatisfactory yield, reaction quenching and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Continuously feed dry nitrogen into the clean reactor, and add 4.86 g (0.2 mol) of magnesium chips, 3.80 g (0.03 mol) of p-chlorotoluene, and 15.33 g of solvent into the reactor. The temperature was raised with stirring, and the reaction was initiated at 42°C.
[0051] The pre-prepared p-chlorotoluene solution (21.52g (0.17mol) p-chlorotoluene, 65.02g 2-methyltetrahydrofuran) was added dropwise, and the temperature was controlled at about 55°C. After adding for 1 hour, cool down to about 25°C after adding and keeping warm for 1 hour.
[0052] Maintain the temperature of the kettle at about 25°C, add the pre-prepared phosphorus trichloride solution (phosphorus trichloride 8.25g (0.18mol), 2-methyltetrahydrofuran 20.21g) dropwise, add it for 1 hour, keep it warm for 1 hour and then cool it down for use .
[0053] Maintain the pressure of about -0.3MPa in the kettle, slowly increase the temperature of the kettle, and the inner temperature reaches about 85°C in about 1 ho...
Embodiment 2
[0057] Dry nitrogen was continuously fed into the clean reactor, and 19.44 g (0.8 mol) of magnesium chips, 11.97 g (0.064 mol) of p-bromoanisole, and 60.33 g of tetrahydrofuran were added to the reactor. The temperature was raised with stirring, and the reaction was initiated at 42°C.
[0058] Add the pre-prepared p-bromoanisole solution (137.66 g (0.736 mol) p-bromoanisole, 260.03 g tetrahydrofuran) dropwise, and control the temperature to about 55°C. After adding for 1 hour, keep warm for 1 hour and cool down for later use.
[0059] Maintain the temperature of the kettle at about 25°C, add the pre-prepared phosphorus trichloride solution (phosphorus trichloride 33.05g, tetrahydrofuran 80.21g) dropwise, add it in 1 hour, keep it warm for 1 hour, and cool it down for use.
[0060] Maintain the pressure of about -0.4MPa in the kettle, slowly increase the temperature of the kettle for about 1.5 hours, and the inner temperature reaches about 85°C. After the precipitation is comp...
Embodiment 3
[0064] The specific operation is the same as in Example 1, except that 0.06 mol of p-chlorotoluene is added in step 1. When the temperature is raised to 42° C., the reaction is very violent, resulting in flushing.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com