Self-plasticizing vinyl chloride-based copolymer, method for producing same, composition comprising same, and resin product produced from same
A technology for plasticizing vinyl chloride and copolymers, which is applied in the field of resin products, its preparation method, its composition, and self-plasticizing vinyl chloride-based copolymers, and can solve the problem of insufficient self-plasticizing and industrial practical value. High, comonomer self-polymerization and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0091]
[0092] The preparation method of the self-plasticizing vinyl chloride copolymer of the present invention is the preparation method of the above-mentioned self-plasticizing vinyl chloride copolymer, which comprises: making the raw material including vinyl chloride and the monomer represented by the above formula (1) Polymerization.
[0093] The details of the monomer represented by the above formula (1) and other optional monomers have been described above and will not be repeated here.
[0094] The production method of the self-plasticizing vinyl chloride copolymer of the present invention is preferably carried out in the presence of a radical polymerization initiator. In other words, the preparation method of the self-plasticizing vinyl chloride copolymer of the present invention is preferably based on a free radical polymerization mechanism, and the preparation method will be described in detail below.
[0095] (polymerization initiator)
[0096] Initiators for ...
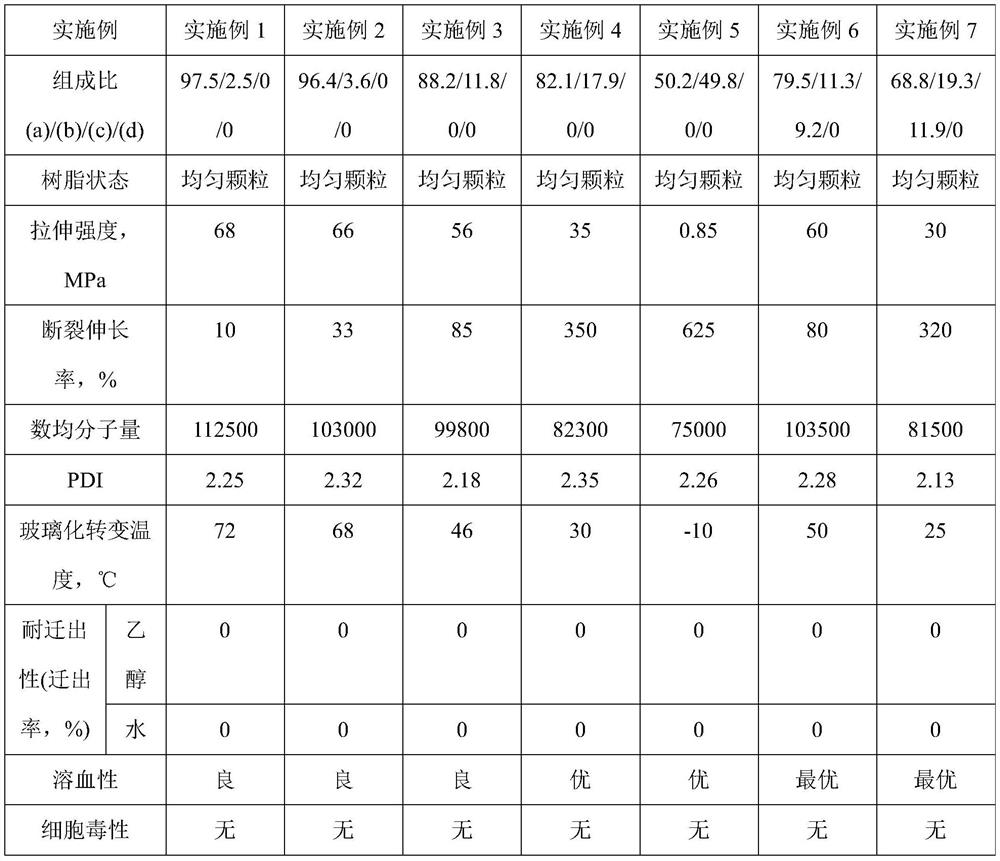
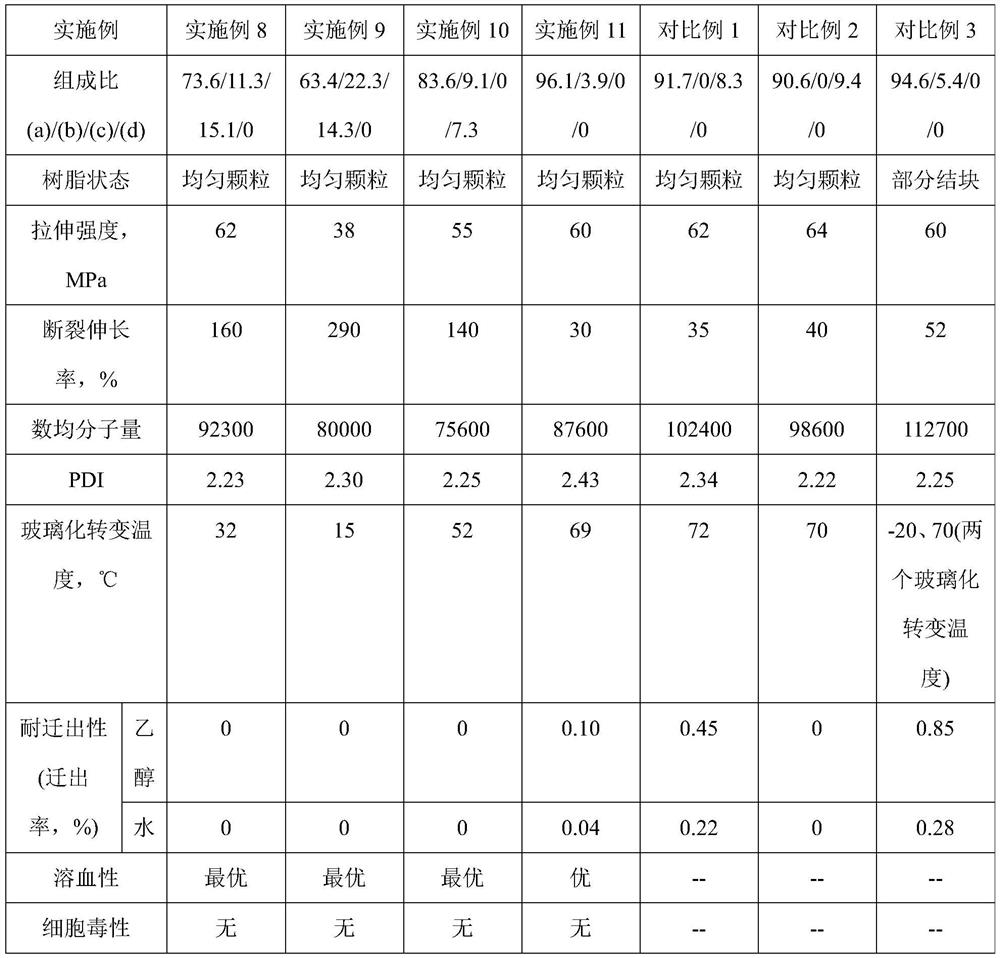
Embodiment 1
[0143] In a stainless steel micro-reactor with an internal volume of 200ml of stirring blades, 100g of deionized water, 10.4g of 2 mass % PVA aqueous solution, 0.042g of hydroxypropylmethylcellulose, 0.05g of azobisisobutyronitrile, 1.62 g of polypropylene glycol monomethacrylate with a molecular weight of 375 (PPGMA-375, the case where x=5 in formula (1)) was filled with nitrogen for 5 minutes to replace the air in the reaction vessel. Then, 52.38 g of VC monomer was introduced into the reactor. After pre-stirring for 60 minutes, the temperature was raised to 70°C to start polymerization. It should be noted that the monomer feeding mass ratio VC:PPGMA-375=97:3, and the polymerization reaction time was 8 hours. After the polymerization reaction was completed, unreacted VC monomer was reclaimed, and the polymerization product was alternately washed with a large amount of deionized water and ethanol to obtain 50.5 g of the vinyl chloride copolymer of white solid particles, and i...
Embodiment 2
[0145] Change the feed mass ratio of VC and PPGMA-375 to be VC:PPGMA-375=95:5 and the polymerization reaction time is 7.5 hours, except that, in the same manner as in Example 1, obtain the vinyl chloride copolymerization of white solid particles 48.2 g of the product, the composition: the content of the structural unit (a) was 96.4% by mass, and the content of the structural unit (b) was 3.6% by mass based on the total mass of the vinyl chloride-based copolymer.
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
| grafting amount | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com