Preparation method of three-dimensional manganese coordination polymer
A coordination polymer, three-dimensional technology, applied in the field of coordination polymers and their preparation, can solve the problems of unsatisfactory stability, high cost, complicated preparation process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] In this embodiment, a preparation method of a three-dimensional manganese coordination polymer, the steps are as follows:
[0033] a. Weigh 0.05 mmol of 5,5'-(1,2-phenylene bis(methoxy))diisophthalic acid ligand material and dissolve it in 1.5 ml of N,N-dimethylformamide , then add 0.05 mmol bpmp nitrogen-containing ligand to obtain a ligand solution;
[0034] b. Weigh 0.20 mmol of manganese nitrate and dissolve it in 7.5 milliliters of deionized water to obtain a manganese salt solution;
[0035] c. the ligand solution prepared in the step a and the manganese salt solution prepared in the step b are mixed in a reaction kettle with a Teflon liner with a volume of 15 milliliters to obtain a mixture of reactants solution; at 85°C, keep the mixed solution of reactants at a constant temperature for 72h for hydrothermal synthesis reaction, then cool down to room temperature with a rate gradient of 10°C / h, and then filter the product solution to obtain light yellow rod-shape...
Embodiment 2
[0037] This embodiment is basically the same as Embodiment 1, especially in that:
[0038] In this embodiment, a preparation method of a three-dimensional manganese coordination polymer, the steps are as follows:
[0039] a. Weigh 0.05 mmol of 5,5'-(1,2-phenylene bis(methoxy))diisophthalic acid ligand material and dissolve it in 1.5 ml of N,N-dimethylformamide , then add 0.05 mmol bpmp nitrogen-containing ligand to obtain a ligand solution;
[0040] b. Weigh 0.20 mmol of manganese nitrate and dissolve it in 7.5 milliliters of deionized water to obtain a manganese salt solution;
[0041] c. the ligand solution prepared in the step a and the manganese salt solution prepared in the step b are mixed in a reaction kettle with a Teflon liner with a volume of 15 milliliters to obtain a mixture of reactants solution; at 85°C, keep the mixed solution of reactants at a constant temperature for 48 hours for hydrothermal synthesis reaction, then cool down to room temperature at a rate o...
Embodiment 3
[0054] This embodiment is basically the same as the previous embodiment, and the special features are:
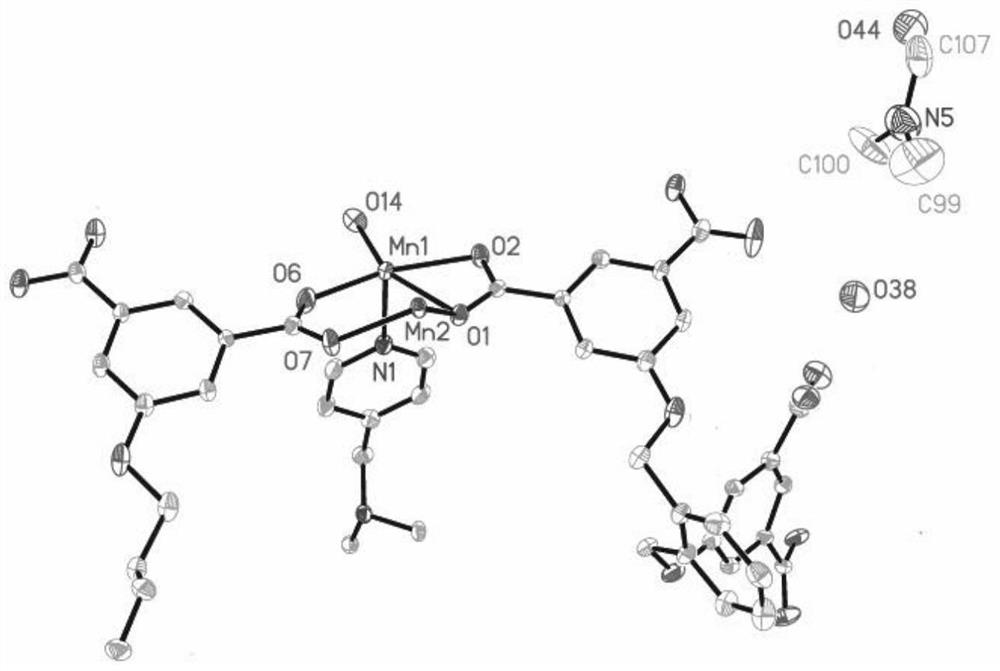
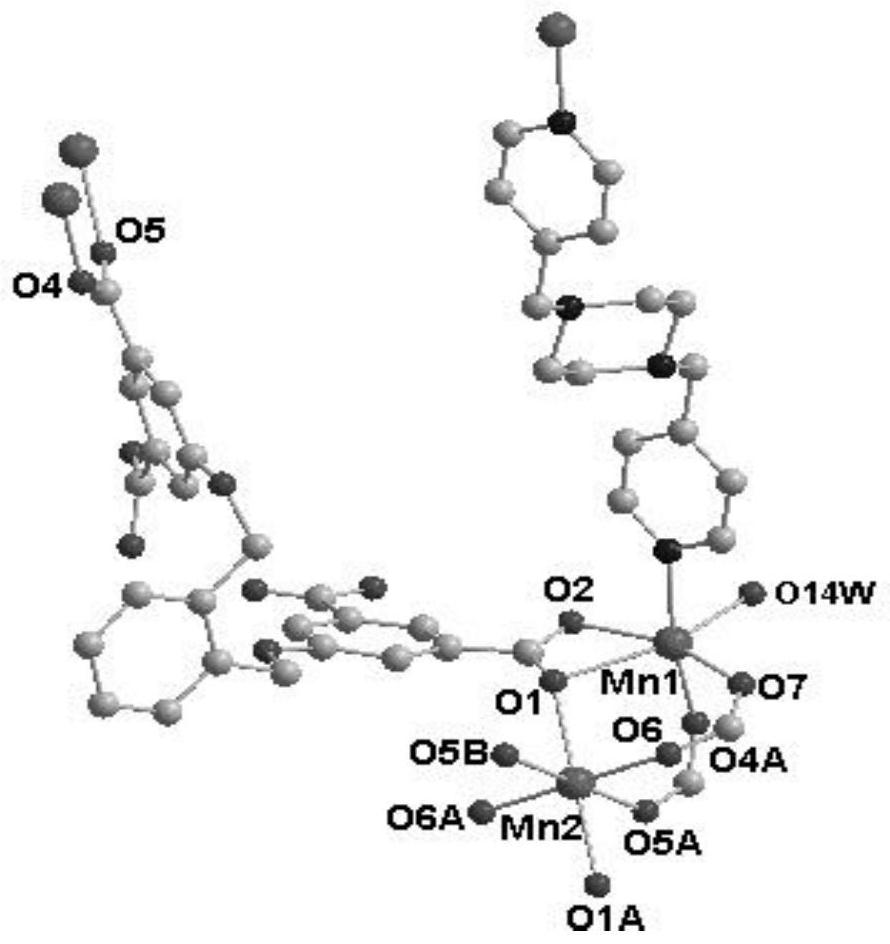
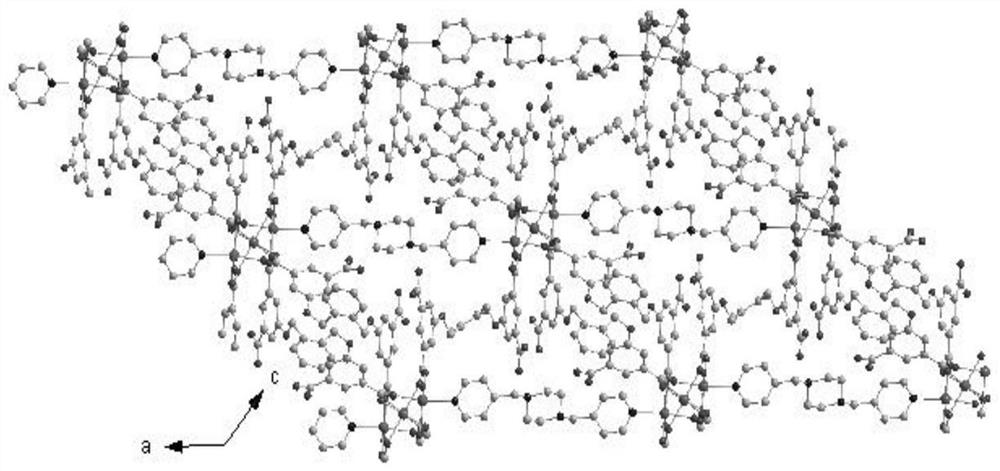
[0055] In this example, see figure 1 ,
[0056] In this embodiment, a preparation method of a three-dimensional manganese coordination polymer, the steps are as follows:
[0057] a. Weigh 0.05 mmol of 5,5'-(1,2-phenylene bis(methoxy))diisophthalic acid ligand material and dissolve it in 1.5 ml of N,N-dimethylformamide , then add 0.05 mmol bpmp nitrogen-containing ligand to obtain a ligand solution;
[0058] b. weigh 0.25 millimoles of manganese nitrate and dissolve it in 7.5 milliliters of deionized water to obtain a manganese salt solution;
[0059] c. the ligand solution prepared in the step a and the manganese salt solution prepared in the step b are mixed in a reaction kettle with a Teflon liner with a volume of 15 milliliters to obtain a mixture of reactants solution; at 100°C, keep the mixed solution of reactants at a constant temperature for 48 hours for hydrothe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com