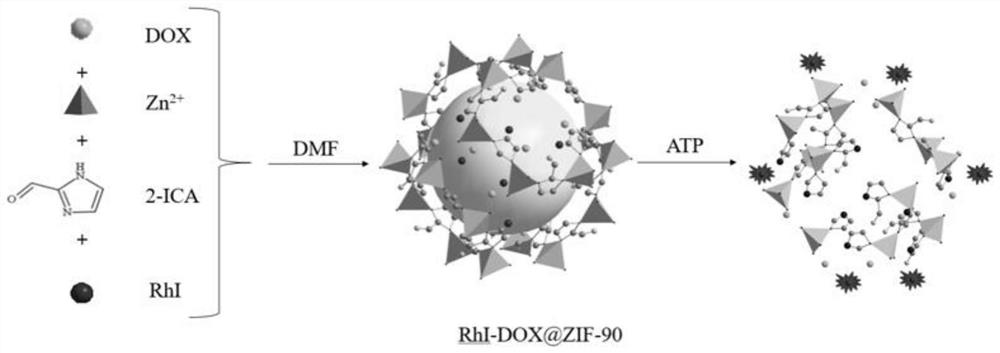
Preparation and application of near-infrared fluorescent probe based on MOF material
A fluorescent probe and near-infrared technology, applied in the field of fluorescent probes, can solve problems such as poor stability, poor tissue targeting, and reduced treatment efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Preparation of fluorescent probes
[0027] Preparation of rhodamine-like near-infrared dye RhI: In a 100mL single-necked flask, 5 equivalents of cyclohexanone was added dropwise to 10mL of concentrated sulfuric acid to cool, then, under stirring, 2.5 equivalents of 2-(4-diethylamino -2-Hydroxybenzoyl)benzoic acid was added to concentrated sulfuric acid, the reaction was heated to 90°C, and the reaction was stirred for 1.5h. It was observed that the reaction solution changed from yellowish brown to black. After stopping the reaction, the resulting reaction product was quickly poured into a beaker filled with crushed ice, and then 2 mL of perchloric acid was added while stirring. After the crude product was vacuum filtered and washed with cold water, the intermediate product was obtained as an orange solid (yield 91%). In a 100mL round bottom flask, put 2 equivalents of the intermediate product, 1 equivalent of Fisher's aldehyde and 8mL of glacial acetic acid in turn, st...
Embodiment 2
[0030] Fluorescent probe and ATP solution preparation
[0031] Preparation of probe solution: Weigh a certain amount of probe and disperse it in water to prepare a 4 mg / mL probe solution. Preparation of ATP solution: Weigh a certain amount of adenosine-5-triphosphate disodium salt and dissolve it in distilled water, configure it into 20mM ATP solution, and store it in an environment at 4°C.
Embodiment 3
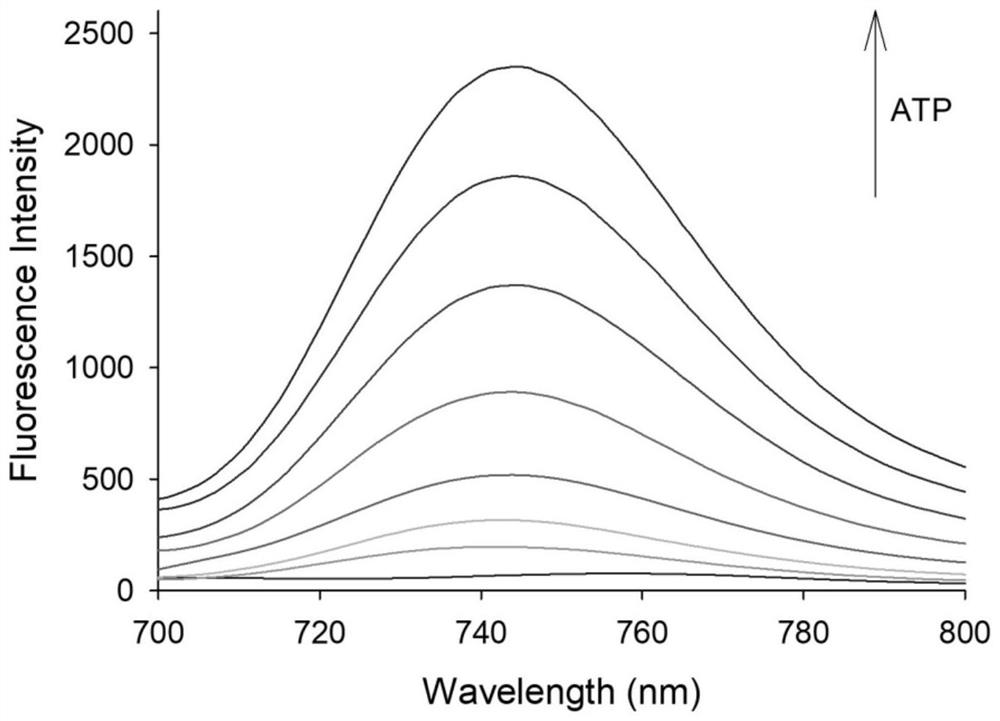
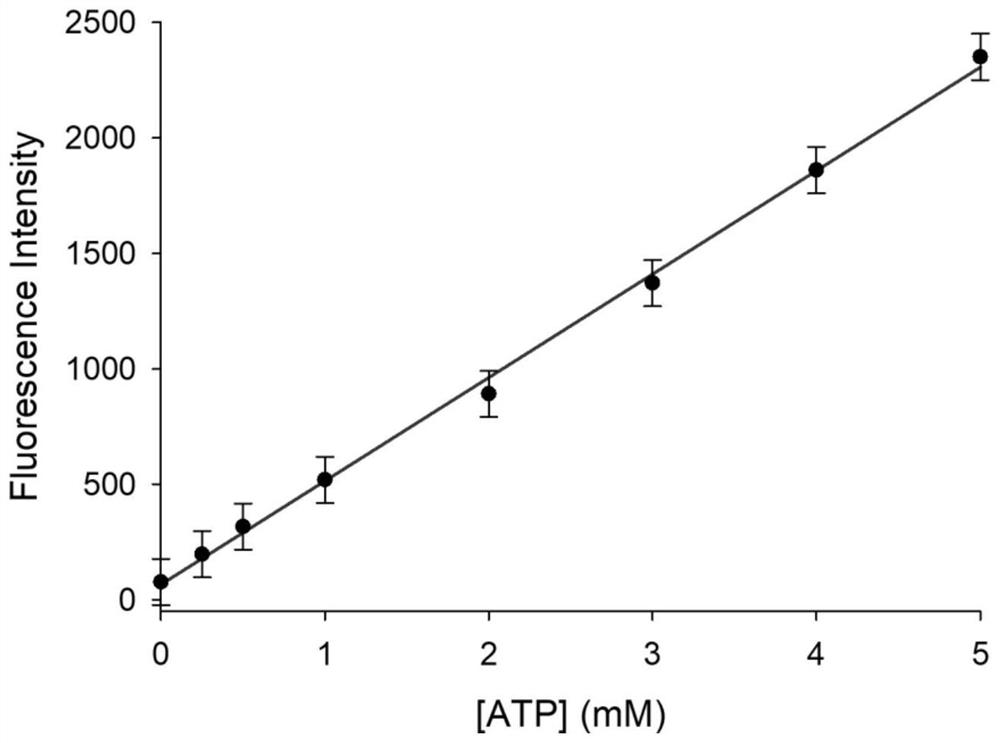
[0033] Measurement of Fluorescence Spectrum of Interaction of Fluorescent Probe with ATP and Measurement of Doxorubicin Release Rate
[0034] figure 2 The fluorescence spectrum of the interaction between the fluorescent probe and ATP, the concentration of the fluorescent probe is 4mg / L, and the concentration of ATP is 0, 0.25, 0.5, 1, 2, 3, 4, 5mM. The excitation wavelength is 680nm and the emission wavelength is 750nm. The slit width is 5.0 nm / 5.0 nm, and the fluorescence measurement instrument used is a Hitachi F4600 fluorescence spectrophotometer. Such as figure 2 As shown, before adding ATP, the probe basically does not emit fluorescence signal; after adding ATP, the probe has an emission peak at 750nm, and the fluorescence intensity increases with the increase of ATP concentration. This is because ATP and Zn in the material 2+ Binding leads to the collapse of the ZIF-90 structure of the probe, thereby releasing the fluorophore RhI and drug DOX wrapped in it. And wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com