Serum-free Vero cell fixed bed bioreactor high-density culture process
A technology of bioreactor and high-density culture, which is applied in the field of cell culture, can solve the problems of reducing culture shear force, achieve the effect of reducing pollution and increasing cell density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
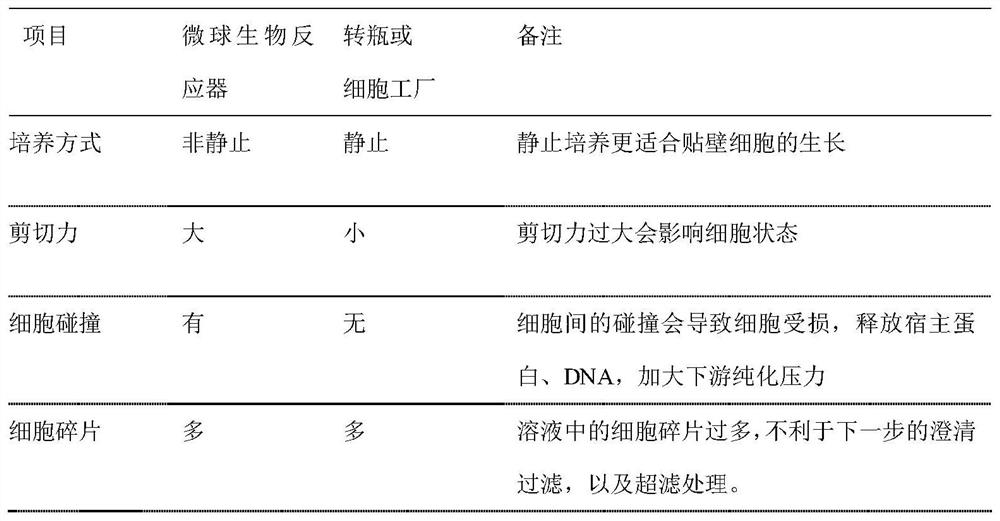
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1 Serum-free Vero cell fixed-bed bioreactor high-density culture
[0031] 1. Production cells
[0032] The cells used for production were Vero cells Vero-SF-ACF adapted to serum-free culture, which were purchased from the American type culture collection (American Type Culture Collection), and the ATCC number was CCL-81.5.
[0033] 2. Method
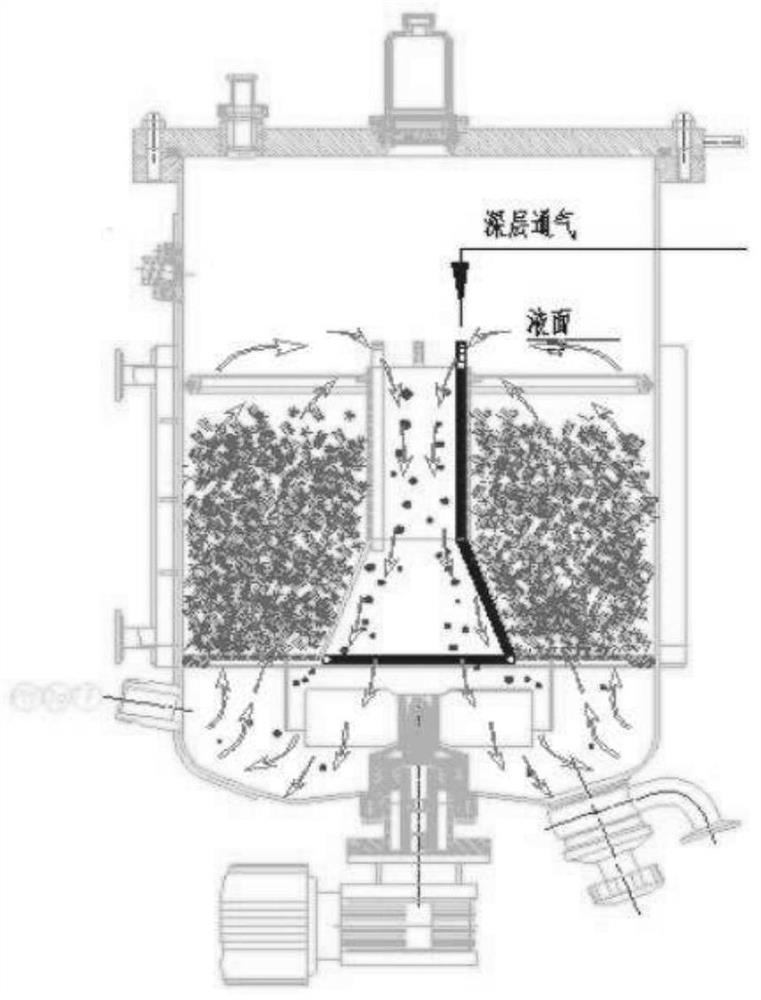
[0034] 1) Fill the sheet carrier (such as figure 1 purple area).
[0035] 2) Place Serum-free Vero cells from the working cell bank in a T75 square flask and culture them with serum-free medium. When they grow into a dense monolayer, use recombinant cell digestion enzyme (instead of animal-derived trypsin) to digest the cells, and press 1: 6 Amplify and passage, digest cells with recombinant cell digestion enzyme, and then inoculate into fixed bed bioreactor, the cell density in the reactor is 6×10 5 cells / ml.
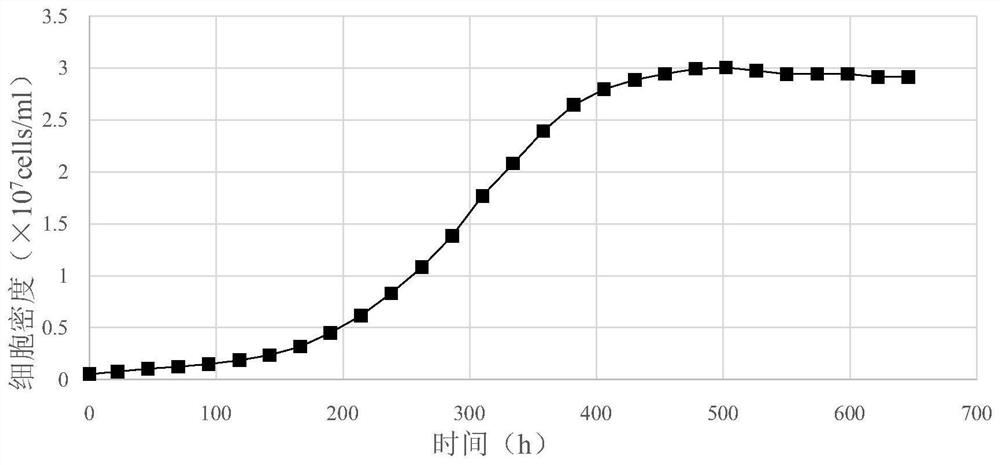
[0036] 3) After the cell suspension completely entered the reactor for 60 minutes, the cell adsorption w...
Embodiment 2
[0043] Embodiment 2 Serum-free Vero cell fixed-bed bioreactor high-density culture
[0044] 1. Production cells
[0045] The cells used for production were Vero cells Vero-SF-ACF adapted to serum-free culture, which were purchased from the American type culture collection (American Type Culture Collection), and the ATCC number was CCL-81.5.
[0046] 2. Method
[0047] 1) In the carrier filling area of the fixed bed bioreactor, fill the sheet carrier (such as figure 1 purple area).
[0048] 2) Place Serum-free Vero cells from the working cell bank in a T75 square flask and culture them with serum-free medium. When they grow into a dense monolayer, use recombinant cell digestion enzyme (instead of animal-derived trypsin) to digest the cells, and press 1: 3 Amplify and passage, digest cells with recombinant cell digestion enzyme, and then inoculate into fixed bed bioreactor, the cell density in the reactor is 5×10 5 cells / ml.
[0049] 3) After the cell suspension completel...
Embodiment 3
[0057] Example 3 Serum-free Vero cells fixed bed bioreactor high density culture
[0058] 1. Production cells
[0059] The cells used for production were Vero cells Vero-SF-ACF adapted to serum-free culture, which were purchased from the American type culture collection (American Type Culture Collection), and the ATCC number was CCL-81.5.
[0060] 2. Method
[0061] 1) Fill the sheet carrier (such as figure 1 purple area).
[0062] 2) Place Serum-free Vero cells from the working cell bank in a T75 square flask and culture them with serum-free medium. When they grow into a dense monolayer, use recombinant cell digestion enzyme (instead of animal-derived trypsin) to digest the cells, and press 1: 6 Amplify and passage, digest the cells with recombinant cell digestion enzymes, and then inoculate them into a fixed bed bioreactor. The cell density in the reactor is 8×10 5 cells / ml.
[0063] 3) After the cell suspension completely enters the reactor for 60 minutes, the cell ads...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cell density | aaaaa | aaaaa |
| Cell density | aaaaa | aaaaa |
| Cell density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com