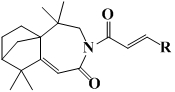
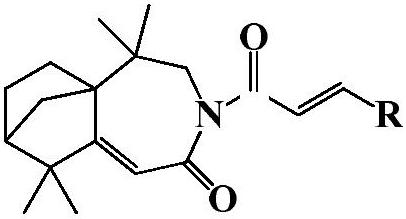
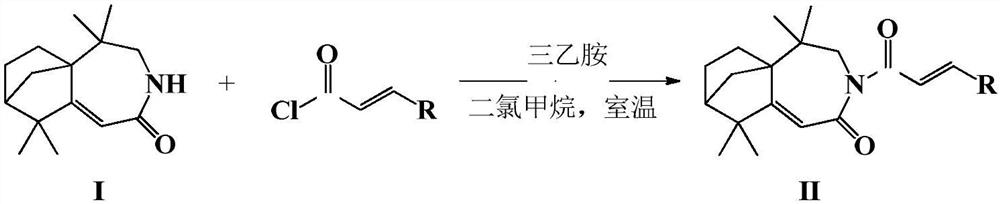
Isophyllenone-caprolactam derivatives and their preparation methods and applications
A ketone compound and tetramethyl technology, applied in the field of isofyl ketone caprolactam derivatives and their preparation, can solve the problems of toxicity accumulation, low antitumor activity and the like, and achieve good inhibitory activity, abundant sources, and synthesis technology. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 3-(4′-fluoro)cinnamoyl-5,5,9,9-tetramethyl-4,5,6,7,8,9-hexahydro-5a,8-methanobenzo[d ] Preparation of azepine-2 (3H)-ketone (compound 1):
[0024]
[0025] Add 5,5,9,9-tetramethyl-4,5,6,7,8,9-hexahydro-5a,8-methanobenzo[d]azepine sequentially into a 50mL one-necked flask In-2(3H)-ketone (0.5mmol), triethylamine (5mmol) and anhydrous dichloromethane (20mL), add p-fluorocinnamoyl chloride (0.5mmol) dropwise under ice bath, and react at room temperature for 4h after the dropwise addition , TLC detected that the reaction was complete, added water, extracted with dichloromethane, combined, concentrated, and purified by column chromatography (petroleum ether:ethyl acetate=4:1) to obtain a white solid powder with a yield of 74.5%. 1 HNMR (600MHz, CDCl 3 )δ: 7.64(d, J=15.5Hz, 1H), 7.54(dd, J=8.6, 5.5Hz, 2H), 7.21(d, J=15.6Hz, 1H), 7.04(t, J=8.6Hz, 2H), 5.74(s, 1H), 4.11(d, J=14.4Hz, 1H), 3.56(d, J=14.4Hz, 1H), 1.97(s, 1H), 1.89-1.91(m, 1H), 1.78-1.83(m, 1H), 1.72(d, J=7....
Embodiment 2
[0027] 3-(4′-methyl)cinnamoyl-5,5,9,9-tetramethyl-4,5,6,7,8,9-hexahydro-5a,8-methanobenzo[ d] Preparation of azepine-2(3H)-one (compound 2):
[0028]
[0029] The preparation method is the same as in Example 1, replacing p-fluorocinnamoyl chloride with p-methylcinnamoyl chloride, and the product 3-(4′-methyl)cinnamoyl-5,5,9,9-tetramethyl-4,5,6, 7,8,9-hexahydro-5a,8-methanobenzo[d]azepin-2(3H)-one is a white solid powder with a yield of 73.8%. 1 H NMR (600MHz, CDCl 3 )δ: 7.68(d, J=15.5Hz, 1H), 7.45(d, J=8.1Hz, 2H), 7.25(d, J=15.5Hz, 1H), 7.16(d, J=7.9Hz, 2H) , 5.74(s, 1H), 4.10(d, J=14.5Hz, 1H), 3.56(d, J=14.5Hz, 1H), 2.35(s, 3H), 1.96(s, 1H), 1.88-1.93( m, 1H), 1.77-1.82(m, 1H), 1.71(d, J=10.2, 1H), 1.51-1.57(m, 1H), 1.39(d, J=10.1Hz, 1H), 1.27-1.32( m, 1H), 1.16(s, 3H), 1.09(s, 3H), 1.05(s, 3H), 0.96(s, 3H); 13 C NMR (151MHz, CDCl 3 )δ:176.42,170.11,170.02,142.90,140.15,132.53,129.44,128.25,120.99,114.25,63.70,49.14,46.07,45.47,37.68,34.34,28.90,28.76,25.37,24.82,24....
Embodiment 3
[0031] 3-(4′-chloro)cinnamoyl-5,5,9,9-tetramethyl-4,5,6,7,8,9-hexahydro-5a,8-methanobenzo[d ] Preparation of azepine-2 (3H)-ketone (compound 3):
[0032]
[0033] The preparation method is the same as in Example 1, replacing p-fluorocinnamoyl chloride with p-chlorocinnamoyl chloride, and the product 3-(4'-chloro)cinnamoyl-5,5,9,9-tetramethyl-4,5,6,7, 8,9-hexahydro-5a,8-methanobenzo[d]azepin-2(3H)-one is a white solid powder with a yield of 77.8%. 1 H NMR (600MHz, CDCl 3 )δ: 7.64(d, J=15.6Hz, 1H), 7.51(d, J=8.5Hz, 2H), 7.34(d, J=8.5Hz, 2H), 7.27(d, J=15.7Hz, 1H) , 5.76(s, 1H), 4.14(d, J=14.5Hz, 1H), 3.58(d, J=14.4Hz, 1H), 1.98(m, 1H), 1.91-1.95(m, 1H), 1.80- 1.84(m, 1H), 1.74(d, J=10.1, 1H), 1.60-1.53(m, 1H), 1.43(d, J=10.2, 1H), 1.30-1.35(m, 1H), 1.19(s , 3H), 1.12(s, 3H), 1.08(s, 3H), 0.98(s, 3H); 13 C NMR (151MHz, CDCl 3 )δ: 176.86, 170.04, 169.71, 141.04, 135.58, 133.81, 129.37, 128.96, 122.68, 114.08, 63.75, 49.12, 46.06, 45.54, 37.67, 34.31, 28.87, 28.79, 24.2.2
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com