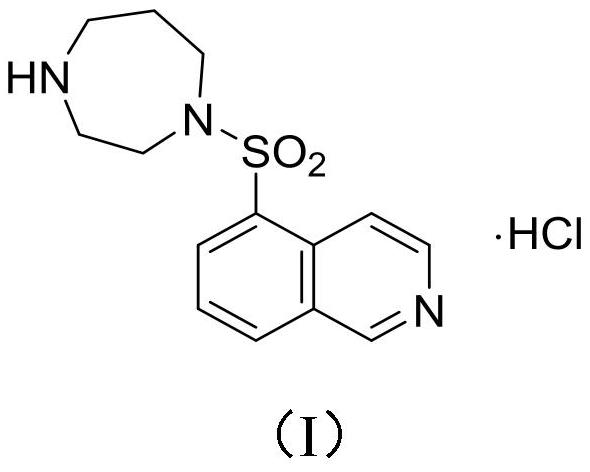
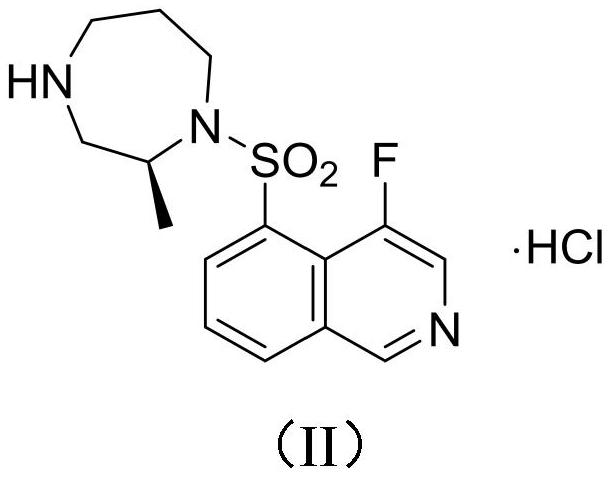
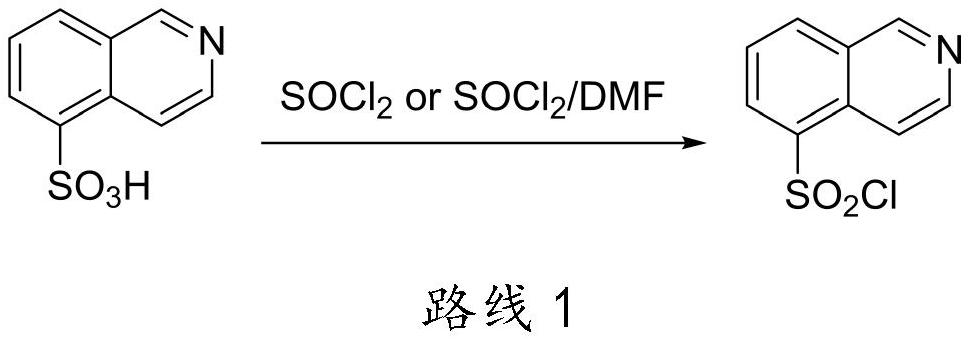
Method for preparing isoquinoline hydrochloride intermediate and rho kinase inhibitor by using btc/ph3po chlorination system
A technology of kinase inhibitor and sulfonyl chloride hydrochloride, which is applied in the field of medicine, can solve the problems of potential safety hazards in storage and transportation, environmental pollution of by-product sulfides, low reaction efficiency of chlorinated reagents, etc. The effect of high product yield and high product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: the synthesis of isoquinoline-5-sulfonyl chloride hydrochloride
[0047] With isoquinoline-5-sulfonic acid (31.38g, 150mmol), BTC (14.84g, 50mmol), Ph 3 PO (2.09g, 7.5mmol) and ethyl acetate (300mL) were added to a 500mL reaction flask, heated to 25°C for 1 hour, cooled down to room temperature, added dichloromethane (150mL) and stirred, and suction filtered to obtain a white solid. Vacuum-dried at 40°C-50°C to obtain 33.68g of solid, with a yield of 85% and an HPLC content of 99.92%. After the filtrate part is concentrated, Ph is precipitated at low temperature 3 PO (precipitation temperature at low temperature is 30°C, the same below), after washing with a small amount of petroleum ether, suction filtration, and drying to obtain Ph 3 PO (1.91g) is reusable.
Embodiment 2
[0048] Embodiment 2: the synthesis of isoquinoline-5-sulfonyl chloride hydrochloride
[0049] With isoquinoline-5-sulfonic acid (31.38g, 150mmol), BTC (14.84g, 50mmol), Ph 3 PO (2.09g, 7.5mmol) and ethyl acetate (300mL) were added to a 500mL reaction flask, heated to 25°C for 5 hours, cooled to room temperature, added dichloromethane (150mL) and stirred, and suction filtered to obtain a white solid. Vacuum-dried at 40°C-50°C to obtain 34.86g of solid, with a yield of 88% and an HPLC content of 99.95%. After the filtrate part is concentrated, Ph is precipitated at low temperature 3 PO, washed with a small amount of petroleum ether, suction filtered, and dried to obtain Ph 3 PO (2.01g) is reusable.
Embodiment 3
[0050] Embodiment 3: the synthesis of isoquinoline-5-sulfonyl chloride hydrochloride
[0051] With isoquinoline-5-sulfonic acid (31.38g, 150mmol), BTC (14.84g, 50mmol), Ph 3 PO (2.09g, 7.5mmol) and ethyl acetate (300mL) were added to a 500mL reaction flask, heated to 25°C for 14 hours, cooled to room temperature, added dichloromethane (150mL) and stirred, suction filtered to obtain a white solid, Vacuum-dried at 40°C-50°C to obtain 34.47g of solid, with a yield of 87% and an HPLC content of 99.94%. After the filtrate part is concentrated, Ph is precipitated at low temperature 3 PO, washed with a small amount of n-hexane, suction filtered and dried to obtain Ph 3 PO (1.89g) is reusable.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com