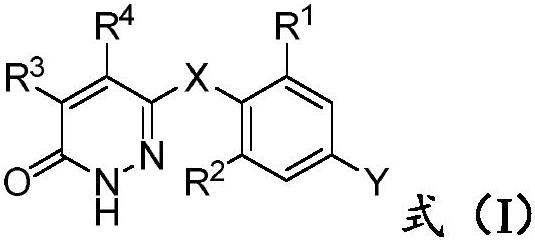
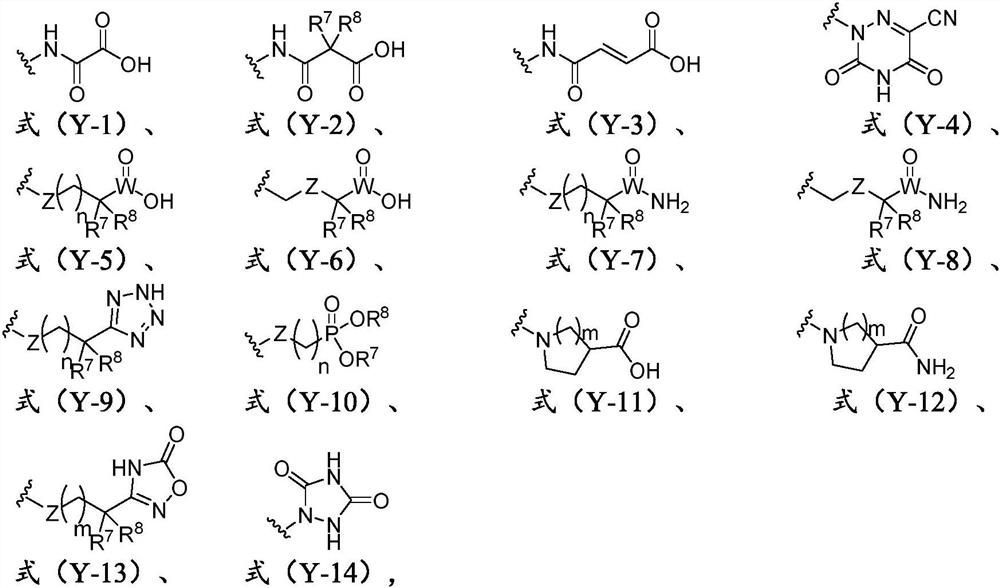
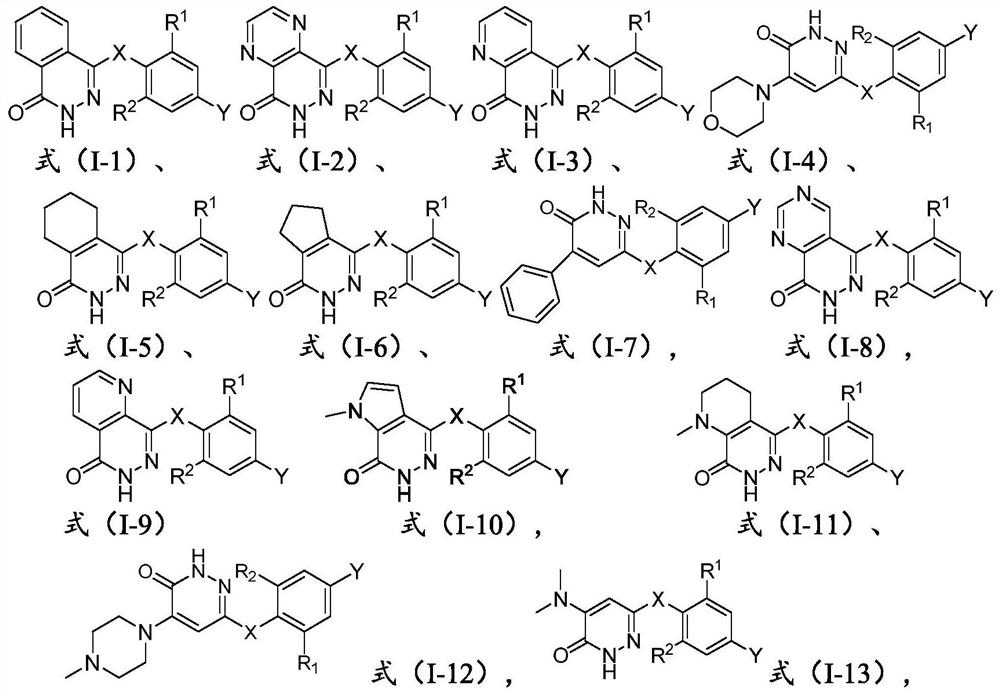
Pyridazinone derivative and application thereof
A kind of derivative, the technology of pyridazinone, applied in the field of pyridazinone derivatives and preparation thereof, can solve the problems such as the limitation of therapeutic use, and achieve the effect of good activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0063] The present invention has no special requirements for the preparation method of the aforementioned pyridazinone derivatives, and those skilled in the art can select a suitable preparation process based on the common knowledge of synthesis based on the target product.
[0064] The present invention also provides an application of the pyridazinone derivatives described in the present invention in the preparation of agonists that stimulate thyroid hormone beta receptors; the pyridazinone compounds of formula (I) provided by the present invention show good Thyroid hormone β receptor agonist has good liver selectivity and can be used as a drug related to the treatment and / or prevention of diseases related to the action.
[0065] Definition of each term:
[0066] It is to be understood that the terminology employed herein is for the purpose of describing particular embodiments and is not intended to be limiting. In addition, although any methods, devices and materials simila...
Embodiment 1
[0075] 3-((3,5-dichloro-4-((5-isopropyl-6-oxo-1,6-dihydropyridazin-3-yl)oxy)phenyl)amino)-3- Malonate
[0076]
[0077] Step 1: Preparation of 3,6-Dichloro-4-isopropylpyridazine (Compound 2)
[0078] Dissolve 3,6-dichloropyridazine (45g, 0.304mol), isobutyric acid (33.45g, 0.380mol), silver nitrate (5.138g, 0.0304mol), trifluoroacetic acid (6.932g, 0.0608mol) in water (270mL) was stirred at 70°C, and ammonium persulfate dissolved in water (180mL) was slowly dropped into the bottle. The reaction solution was stirred for 20 minutes, the heating was stopped, and the temperature was cooled to room temperature. with NaHCO 3 Adjust the pH value of the aqueous solution to 9-10. Extract with n-hexane, combine the organic phases to dry over anhydrous sodium sulfate, filter, remove the solvent under reduced pressure to obtain a residue, and then separate by column chromatography [ethyl acetate / petroleum ether=1 / 3] to obtain 3,6-dichloro-4 - Isopropylpyridazine (56 g, 96% yield)....
Embodiment 2
[0093] (E)-4-((3,5-dichloro-4-((5-isopropyl-6-oxo-1,6-dihydropyridazin-3-yl)oxy)phenyl)amino )-4-oxo-2-butenoic acid
[0094]
[0095] Step 1: Preparation of (E)-4-((3,5-dichloro-4-((6-chloro-5-isopropylpyridazin-3-yl)oxy)phenyl)amino)-4- Oxo-2-butenoic acid methyl ester (compound 6)
[0096] 3,5-dichloro-4-((6-chloro-5-isopropylpyridazin-3-yl)oxy)aniline (100mg, 0.301mmol), triethylamine (60mg, 0.602mmol) into dichloro Methane (3mL), ice bath, then slowly drop methyl 3-chlorocarbonylacrylate (49mg, 0.331mmol) into the bottle, react at room temperature for 20 minutes, add water to quench the reaction, extract with ethyl acetate, combine the organic phases, Dry over anhydrous sodium sulfate, filter, and remove solvent under reduced pressure to obtain (E)-4-((3,5-dichloro-4-((6-chloro-5-isopropylpyridazin-3-yl)oxy )phenyl)amino)-4-oxo-2-butenoic acid methyl ester (140 mg, 104% yield).
[0097] LC-MS (m / z): 444.5 (M+1).
[0098] Step 2: Preparation of (E)-4-((3,5-dichloro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com