Preparation method of biphenyl tetradentate phosphite ligand
A technology of biphenyl tetradentate phosphite and biphenyl tetradentate phosphite, which is applied in the field of preparation of biphenyl tetradentate phosphonite ligands and can solve the problem of high production cost of 1-butene
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Preparation of 2,2’,6,6’-tetramethoxy-1,1’-biphenyl:
[0020]
[0021] Add 1 (13.8g), THF (200mL), tetramethylethylenediamine (16 g) in sequence to a 1L three-neck flask, and add n-butyllithium (40mL 2.5mol / L ), then add ferric chloride (16.1g), after addition completes, reaction bottle is heated to room temperature reaction 24 hours. The solvent was spin-dried under reduced pressure, 400 mL of water was added, and extracted three times with ethyl acetate (500 mL each time). The obtained organic phase was dried over anhydrous sodium sulfate and then spin-dried under reduced pressure, and the residue was subjected to flash column chromatography to obtain 22 g of the target product with a yield of 80%.
Embodiment 2
[0023] Preparation of 2,2’,6,6’-tetrahydroxy-1,1’-biphenyl:
[0024]
[0025] In a 1L four-neck round-bottom flask, add 2 (27g) and 500mL of dichloromethane in sequence, drop boron tribromide (101g) at -30°C, and raise the resulting reaction system to 30°C to react 4 Hour. After the resulting reaction mixture was concentrated, 400 mL of water was added and extracted three times with ethyl acetate (600 mL each time). The residue was subjected to column chromatography to obtain 20.5 g of the target product with a yield of 91%.
Embodiment 3
[0027] Preparation of 2,2',6,6'-tetramethoxy-3,3',5,5'-tetra-tert-butyl-1,1'-biphenyl:
[0028]
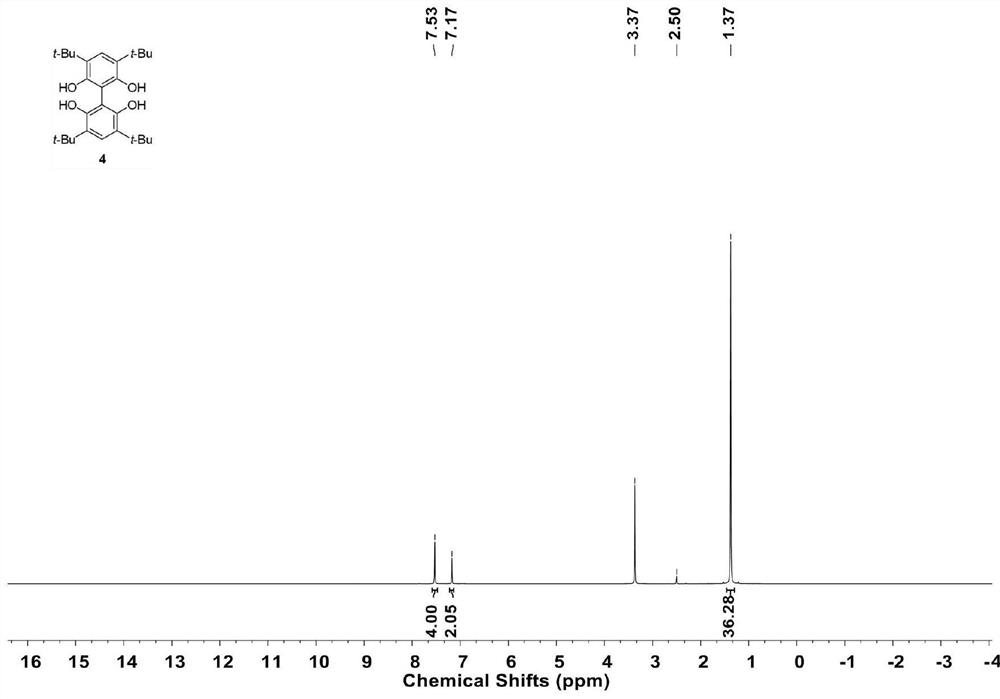
[0029] Add 3 (22.0 g) to a dry 1 L Schlenk bottle, replace the reaction bottle with a nitrogen atmosphere, and add 200 mL of tetrahydrofuran, organic or inorganic acid (1.0 g) at 25°C. Isobutene at a pressure of 1.5 atmospheres was continuously fed, and the reaction was continued for 12 hours. After the reaction solution was quenched with water, 300 mL of water was added and extracted three times with ethyl acetate (400 mL each time). The obtained organic phase was dried over anhydrous sodium sulfate, and then spin-dried under reduced pressure to obtain a light yellow solid. Column chromatography obtained 40.0 g of the target product with a yield of 90%. 1 H NMR (600MHz, (CD 3 ) 2 SO): δ=7.53(s,4H), 7.17(s,2H), 1.37(s,36H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com