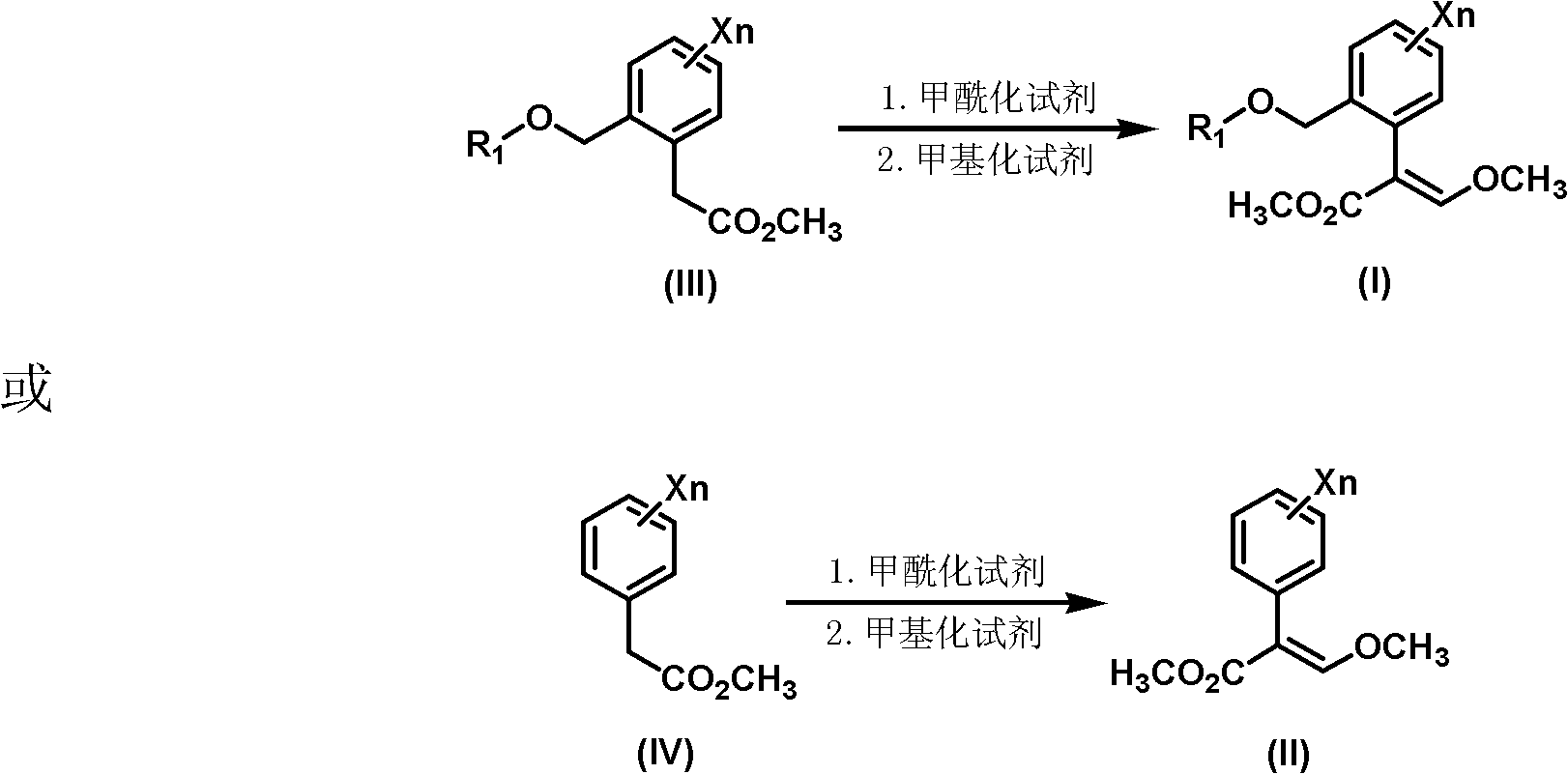
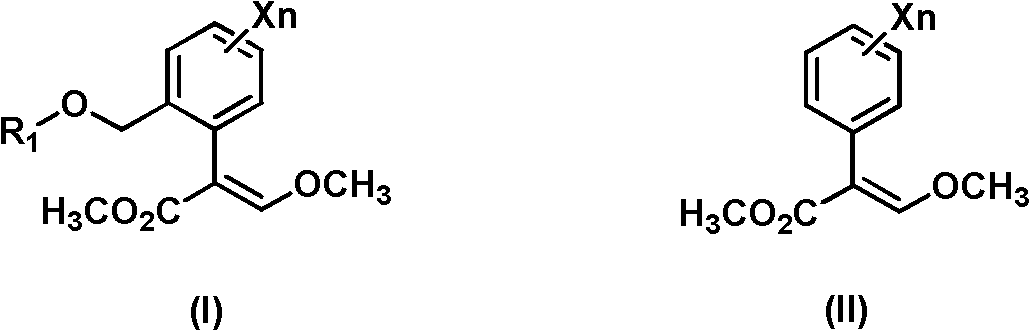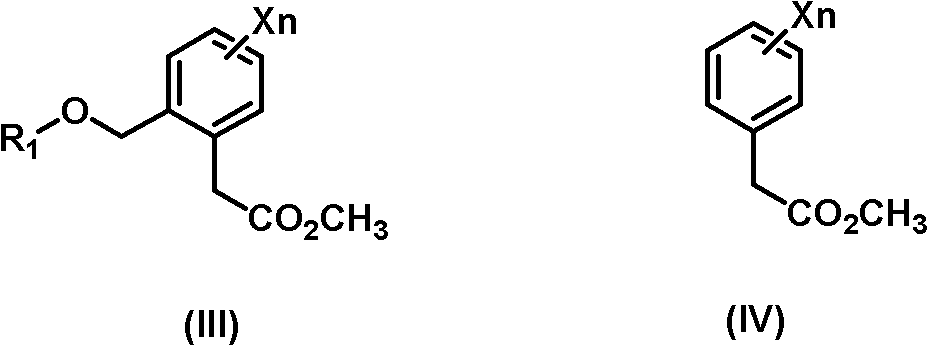Synthesis method of 3-methoxy-2-aryl(methyl)acrylate compounds
A technique for the synthesis of aromatic methyl acrylate, which is applied in the preparation of organic compounds, chemical instruments and methods, and preparation of carboxylic acid esters. Amplified, easy-to-control, low-cost effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Preparation of (E)-methyl 3-methoxy-2{2-[6-(trifluoromethyl)-2-pyridyloxymethyl]phenyl}acrylate.
[0032] Under the protection of dry nitrogen, dichloromethane (350ml) and titanium tetrachloride (58.1g, 0.3mol) were successively added to the reaction flask, the temperature was lowered to 10°C, and trimethyl orthoformate (31.9 g, 0.3mol). After stirring at room temperature for 1 h, the reaction solution was cooled to 0° C., and methyl 2-[6-(trifluoromethyl)-2-pyridyloxymethyl]phenylacetate (97.6 g, 0.3 mol) was added dropwise. After stirring at room temperature for 2 h, the reaction solution was cooled to 5° C., and triethylamine (61.9 g, 0.6 mol) was added. After stirring at room temperature for 1 h, the reaction solution was cooled to 10° C., and 10% dilute hydrochloric acid (219.2 g, 0.6 mol) was added dropwise. After 10 min, add 200 mL of water to wash, separate the layers, and take the organic layer as the intermediate.
[0033] At room temperature, benzyltriethy...
Embodiment 2
[0035] Preparation of (E)-methyl 3-methoxy-2{2-[6-(trifluoromethyl)-2-pyridyloxymethyl]phenyl}acrylate.
[0036] Under the protection of dry nitrogen, 1,2-dichloroethane (350ml) and titanium tetrachloride (87.1g, 0.45mol) were successively added to the reaction flask, the temperature was lowered to 10°C, and orthoformic acid was added dropwise under vigorous stirring Trimethyl ester (47.8 g, 0.45 mol). After stirring at room temperature for 1 h, the reaction solution was cooled to 0° C., and methyl 2-[6-(trifluoromethyl)-2-pyridyloxymethyl]phenylacetate (97.6 g, 0.3 mol) was added dropwise. After stirring at room temperature for 2 h, the reaction solution was cooled to 5° C., and triethylamine (74.3 g, 0.72 mol) was added. After stirring at room temperature for 1 h, the reaction solution was cooled to 10° C., and 10% dilute hydrochloric acid (263 g, 0.72 mol) was added dropwise. After 10 min, add 200 mL of water to wash, separate the layers, and take the organic layer as the...
Embodiment 3~10
[0039] Preparation of (E)-methyl 3-methoxy-2{2-[6-(trifluoromethyl)-2-pyridyloxymethyl]phenyl}acrylate under different reaction conditions.
[0040] According to the method of Example 2, prepare (E)-3-methoxy-2{2-[6-(trifluoromethyl)-2-pyridyloxymethyl]phenyl} under the following different reaction reagent conditions Methyl acrylate, the results obtained are listed in Table 1:
[0041] Preparation of (E)-3-methoxy-2{2-[6-(trifluoromethyl)-2-pyridyloxymethyl]phenyl}methyl acrylate under different reaction reagent conditions in Table 1
[0042]
[0043] According to the method of Example 2, (E)-3-methoxy-2{2-[6-(trifluoromethyl)-2-pyridyloxymethyl]phenyl}acrylic acid was prepared at the following different reaction temperatures Methyl ester, the obtained results are listed in Table 2:
[0044] Preparation of (E)-3-methoxy-2{2-[6-(trifluoromethyl)-2-pyridyloxymethyl]phenyl}methyl acrylate at different reaction temperatures in Table 2
[0045]
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com