Method for preparing enamine ketone compound
A technology of ketone compounds and compounds, which is applied in the preparation of enaminone compounds with two substituents on the nitrogen atom, in the field of preparation of enaminone compounds, to achieve the effects of mild reaction conditions, improved production efficiency, and easy separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
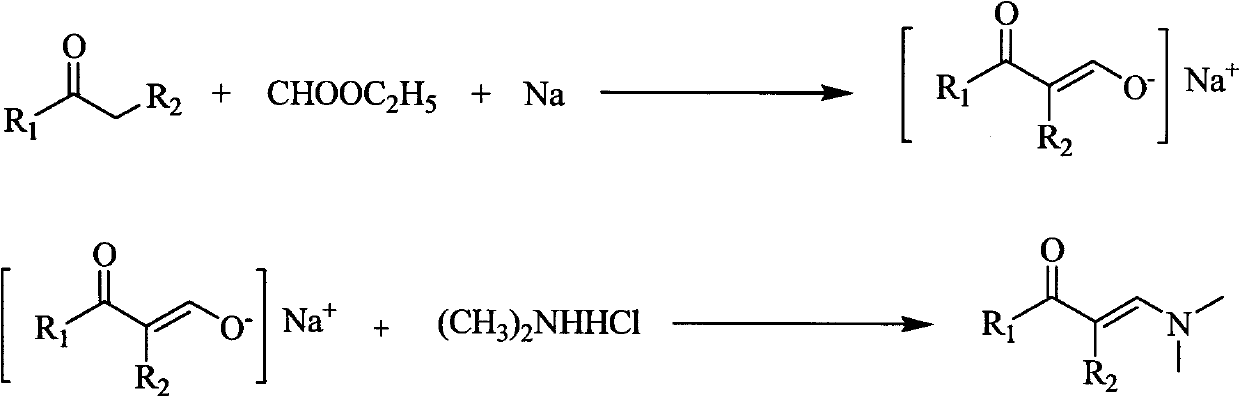
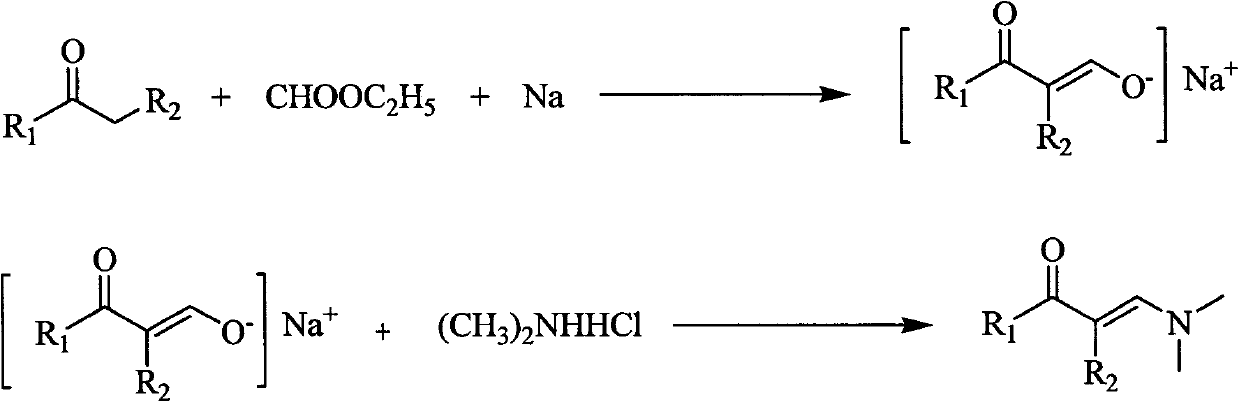
[0029] Preparation of 1-dimethylamino-1-buten-3-one:
[0030] Suspend 4.6g (0.2mol) of sodium metal strips in 100ml of anhydrous diethyl ether, add dropwise a solution consisting of 14.7ml (0.2mol) of acetone and 32ml (0.4mol) of ethyl formate at room temperature, the dropwise addition is completed in about 30 minutes, and continue at room temperature Stir for 5h. Add dropwise 70ml (0.245mol) of dimethylamine hydrochloride aqueous solution with a concentration of 3.5mol / L to the obtained enol sodium salt suspension at room temperature, and the dropwise addition is completed in about 30 minutes, continue stirring at room temperature for 2 hours, and let stand to separate the liquids , the aqueous layer was washed with 3 × 30ml of ether, the organic layers were combined, the organic layer was dried overnight with anhydrous magnesium sulfate, the solvent was removed by rotary evaporation, and the oil pump vacuum distillation gave 17.5ml of light yellow liquid (101-102°C, 5mmHg), ...
Embodiment 2
[0032] Preparation of 1-dimethylamino-2-methyl-1-buten-3-one
[0033] Suspend 4.6g (0.2mol) of sodium metal strips in 100ml of anhydrous ether, add dropwise a solution consisting of 18ml (0.2mol) of methyl ethyl ketone and 32ml (0.4mol) of ethyl formate at room temperature, the dropwise addition is completed in about 30 minutes, and continue at room temperature Stir for 5h. Add dropwise 70ml (0.245mol) of dimethylamine hydrochloride aqueous solution with a concentration of 3.5mol / L to the obtained enol sodium salt suspension at room temperature, and the dropwise addition is completed in about 30 minutes, continue stirring at room temperature for 2 hours, and let stand to separate the liquids , the aqueous layer was washed with 3 × 30ml of ether, the organic layers were combined, the organic layer was dried overnight with anhydrous magnesium sulfate, the solvent was removed by rotary evaporation, and the oil pump decompression distillation gave 15ml of light yellow liquid (80-8...
Embodiment 3
[0035] Preparation of 1-dimethylamino-2-methyl-1-hexen-3-one
[0036] Suspend 4.6g (0.2mol) of sodium metal strips in 100ml of anhydrous ether, add dropwise a solution consisting of 21.5ml (0.2mol) of 3-pentanone and 32ml (0.4mol) of ethyl formate at room temperature, and complete the dropwise addition in about 30 minutes , Stirring was continued for 5h at room temperature. Add dropwise 70ml (0.245mol) of dimethylamine hydrochloride aqueous solution with a concentration of 3.5mol / L to the obtained enol sodium salt suspension at room temperature, and the dropwise addition is completed in about 30 minutes, continue stirring at room temperature for 2 hours, and let stand to separate the liquids , the aqueous layer was washed with 3 × 30ml of ether, the organic layers were combined, the organic layer was dried overnight with anhydrous magnesium sulfate, the solvent was removed by rotary evaporation, and the oil pump decompression distillation gave 24ml of light yellow liquid (102-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com