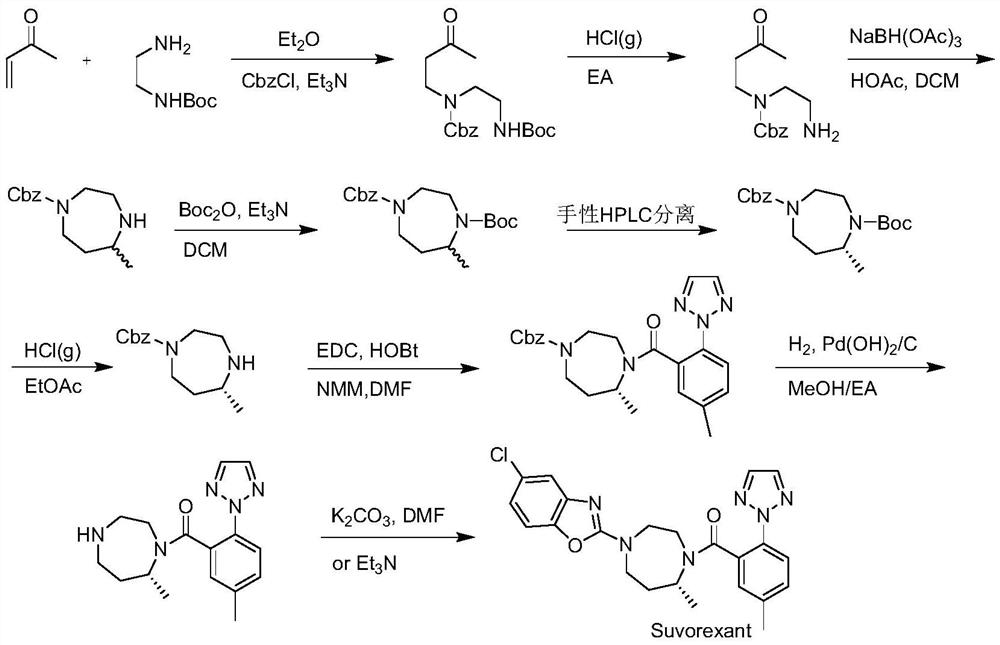
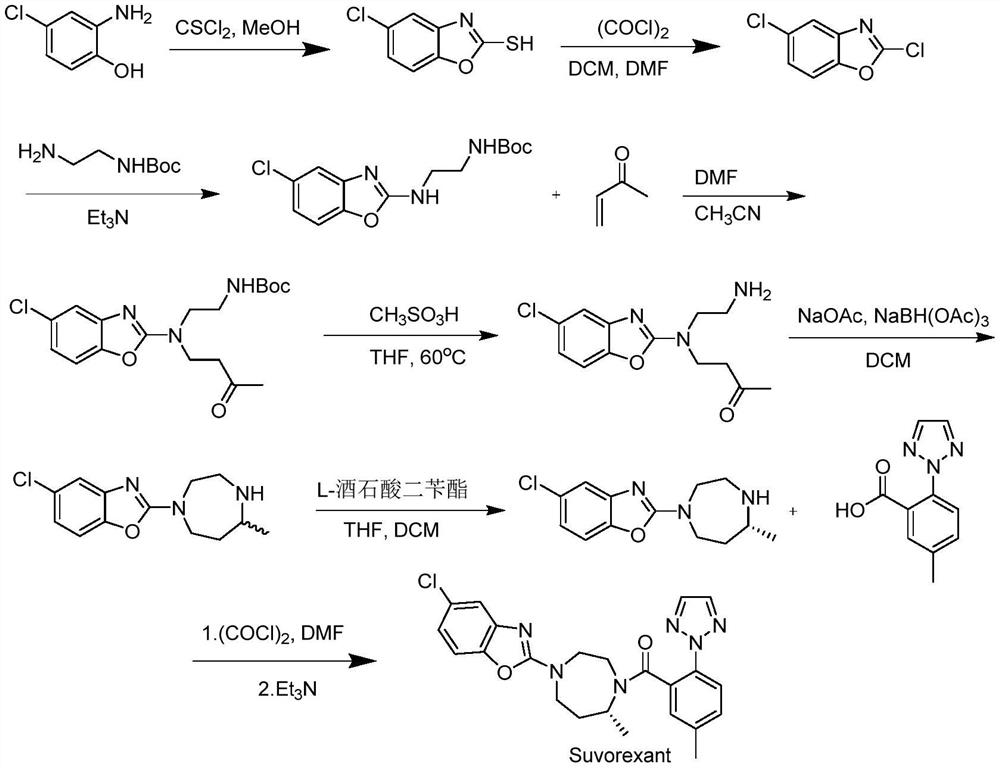
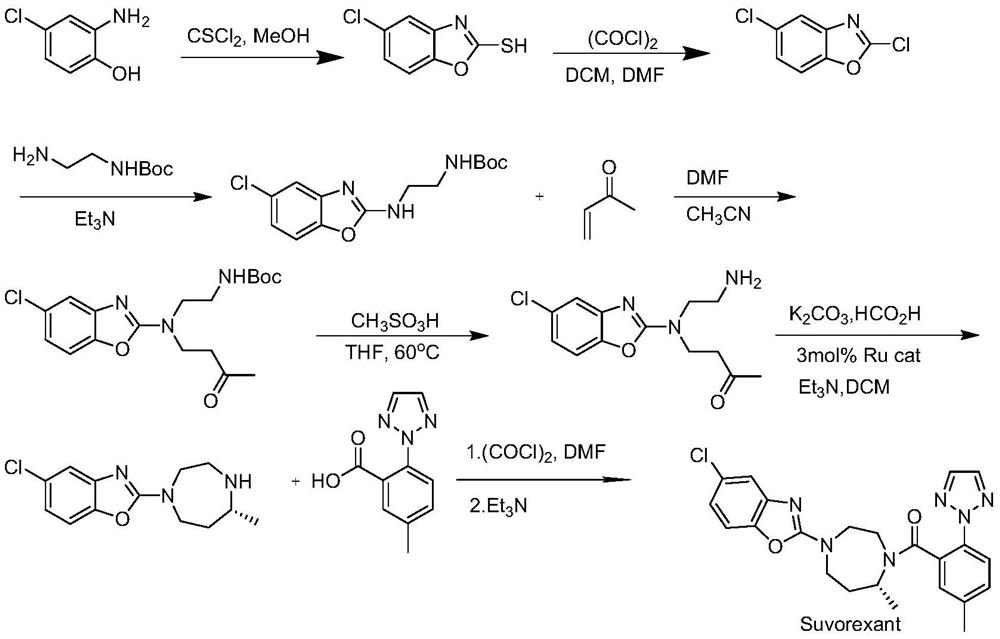
Suvorexant intermediate and preparation method thereof
A technology of intermediates and compounds, which is applied in the fields of organic chemistry and drug synthesis, can solve problems such as irritation, expensive enzymes, and death, and achieve the effects of easy industrialization, simple post-processing, and high ee value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Synthesis of (S)-3-(((2-((tert-butoxycarbonyl)amino)ethyl)((R)-1-phenylethyl)amino)butanoic acid methyl ester:
[0047]
[0048] Under nitrogen protection, (S)-tert-butyl (2-(((1-phenylethyl)amino)ethyl)carbamate (5.8g, 22mmol) was dissolved in 80mL THF and cooled to 0 ℃. Slowly add 1.6M n-butyllithium hexane solution into the system (30.0mL, 44mmol). The resulting solution was stirred for 30 minutes, then cooled to -78℃, then added dropwise in 20mL of anhydrous Methyl crotonate solution (2.0 g, 20 mmol) in THF. The mixture was stirred at -78 ° C for 1 h 30 min. After the reaction was complete, the reaction was quenched, and 20 mL of saturated NH 4 Cl aqueous solution, and the resulting solution was slowly warmed to room temperature. The system was extracted with (2×10 mL) EA. The resulting organic phases were combined and dried with anhydrous MgSO4, then distilled and purified under reduced pressure to obtain 6.5 g of a colorless liquid with a yield of 89%.
[00...
Embodiment 2
[0052] Synthesis of (S)-3-(((2-((tert-butoxycarbonyl)amino)ethyl)((R)-1-phenylethyl)amino)butanoic acid methyl ester:
[0053] Under nitrogen protection, (S)-tert-butyl (2-(((1-phenylethyl)amino)ethyl)carbamate (5.8g, 22mmol) was dissolved in 80mL THF and cooled to 0 ℃. 2.0M tetrahydrofuran solution of lithium diisopropylamide was slowly added to the system (22.0mL, 44mmol), the resulting solution was stirred for 30 minutes, then cooled to -78℃, then added dropwise in 20mL of Methyl crotonate solution (2.0 g, 20 mmol) in water THF. The mixture was stirred at -78 ° C for 1 h 30 min. After the reaction was complete, the reaction was quenched, and 20 mL of saturated NH 4 Cl aqueous solution, and the resulting solution was slowly warmed to room temperature. The system was extracted with (2×10 mL) EA. The obtained organic phases were combined and washed with anhydrous MgSO 4 After drying, it was distilled under reduced pressure and purified to obtain 5.6 g of colorless liquid wi...
Embodiment 3
[0055] Synthesis of (R)-7-methyl-1-((R)-1-phenylethyl)-1,4-diaza-5-lactam:
[0056]
[0057] The Boc protecting group was first removed: the substrate (3.64 g, 10 mmol) was dissolved in 20 mL of DCM, 20 mL of saturated HCl / EA was added, and the reaction was stirred for 6 h. After the reaction was complete, the solvent was removed by rotary evaporation, and the residue was basified with saturated aqueous NaHCO3 and extracted with DCM. Concentrate the organic phase with anhydrous MgSO 4 dry. A de-Boc-protected substrate was obtained. The resulting substrate was dissolved in 40 mL of MeOH under N 2 Join CH under protection 3 ONa (0.65 g, 12 mmol), and stirred at room temperature for 12 h. The reaction was cooled to room temperature and washed with saturated NH 4 Cl aqueous solution was quenched, and then the reaction system was poured into a solution containing 5wt% Na 2 CO 3 In the separatory funnel of the aqueous solution, shake it well and extract it with DCM three ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com