Preparation method of DOTATATE
A technology of -OH and resin, which is applied in the field of DOTATATE preparation, can solve the problems of unfavorable large-scale industrial production, difficult reaction control, unsuitable for industrial production, etc., and achieves easy control of the quality of linear peptides, cheap and easy-to-obtain starting materials, and inhibitory The effect of generation of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
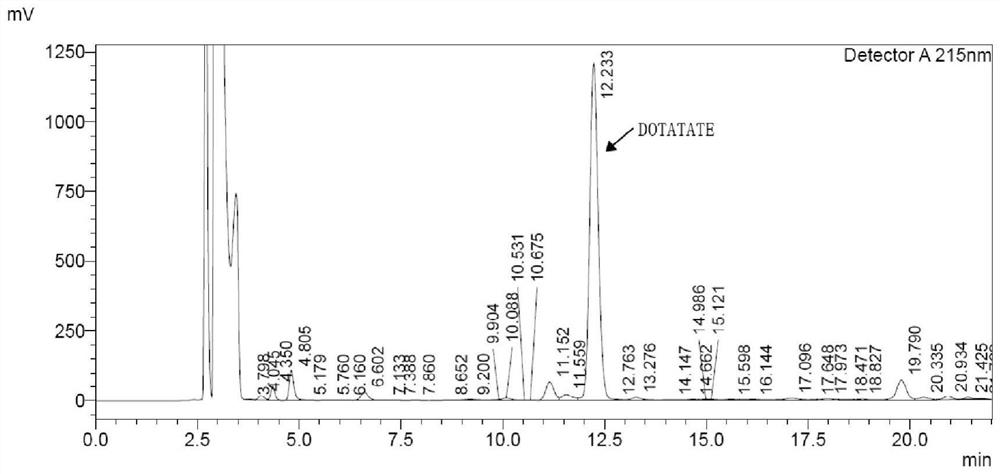
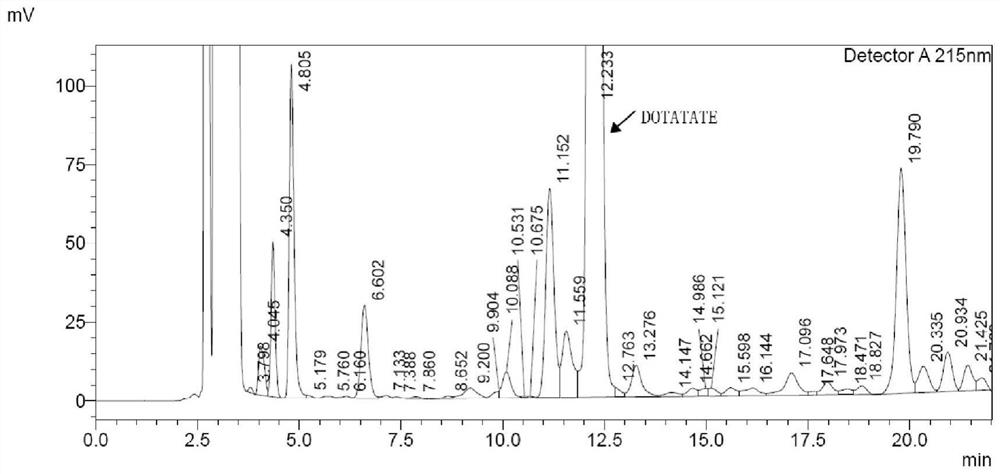
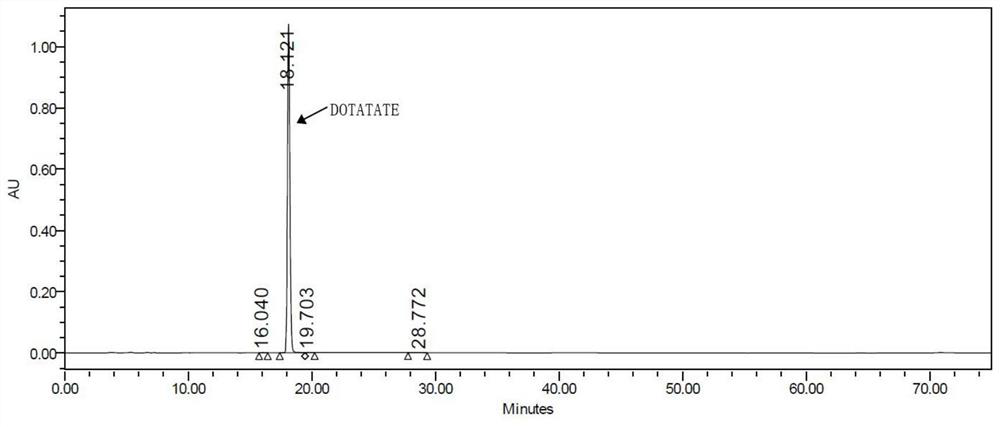
Image
Examples
Embodiment 1
[0025] Step 1: Synthesis of DOTATATE Peptide Resin
[0026] 1) Synthesis of Fmoc-Thr(tBu)-Wang Resin: Weigh 210.02g of Wang Resin (the degree of substitution is 0.96mmol / g, the feeding scale is 201.62mmol), add it to the solid-phase synthesis column, and wash with DMF (1.30L) Once, drained, added DMF (1.30L) to swell for 30min, and drained the DMF.
[0027] Weigh Fmoc-Thr(tBu)-OH (240.43g) and HOBt (98.08g), dissolve with 0.30L DMF, freeze at 0-5°C for 30min, add DIC (114.52g) for activation for 3min; add the above-mentioned swelling resin to the activation solution , additional DMAP (7.40 g) and DMF (0.20 L) were added. Coupling time 4h. After coupling, wash 4 times with DMF (4*1.30L), wash 4 times with DCM (4*50ml), and dry at room temperature. Take the dried peptide resin, vacuum-dry (30min, 30°C, -0.98MPa), measure the degree of substitution of the resin, and the degree of substitution is 0.53mmol / g.
[0028] Wash all the Fmoc-Thr(tBu)-Wang Resin (the degree of substit...
Embodiment 2
[0035] Step 1: Synthesis of DOTATATE Peptide Resin
[0036] 1) Synthesis of Fmoc-Thr(tBu)-CTC Resin: Weigh 210.00g of 2-Cl-CTC resin (the degree of substitution is 0.97mmol / g, the feeding scale is 203.70mmol), add it to the solid-phase synthesis column, and use DMF ( 1.30L) was washed once, drained, added DMF (1.30L) to swell for 30min, and drained the DMF.
[0037] Fmoc-Thr(tBu)-OH (242.89g) was weighed and dissolved in 0.30L DMF, added to the above swelling resin, and then DIEA (157.96g) was added, and the coupling time was 6h. After coupling, wash 4 times with DMF (4*1.30L), wash 4 times with DCM (4*50ml), and dry at room temperature. Take the dried peptide resin, vacuum-dry (30min, 30°C, -0.98MPa), measure the degree of substitution of the resin, and the degree of substitution is 0.48mmol / g.
[0038] Wash all the Fmoc-Thr(tBu)-CTC Resin (the degree of substitution is 0.48mmol / g, the feeding scale is 121.68mmol) with DMF (1.30L), drained, add DMF (1.30L), CH 3 OH (13.04g...
Embodiment 3
[0049] Cleavage of DOTATATE peptide resin: Prepare TFA / TIS / MPR system lysate (1000ml, TFA / TIS / MPR=94 / 4 / 2) with TFA frozen at -10°C, stir well; 100g of the peptide resin obtained in 2 was slowly added to the above lysate, stirred, and cleaved at 20-30°C for 2 hours, the reaction solution was filtered with a dry sand core funnel, the resin was washed 3 times with TFA, the filtrate was collected, and the filtrate was distilled under reduced pressure (- 0.090MPa, 30.0°C, 40min); slowly add the remaining lysate (410ml) into the frozen, stirring isopropyl ether (4.1L), the solid precipitates, stir well after adding, let stand for 30min, separate the liquid, The lower layer was centrifuged, the solid was washed 4 times with isopropyl ether, and the solid was dried in vacuum (-0.98MPa, 30.0°C, 4h) to obtain a linear peptide (38.2g) with a HPLC purity of 55.8% and a yield of 106%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com