Remdesivir liquid preparation for atomizer, and preparation method and application of remdesivir liquid preparation
A liquid preparation, the technology of Remdesivir, applied in the field of medicine, can solve the problems of low bioavailability, large toxic and side effects, slow onset of action, etc., and achieve the effects of small toxic and side effects, mild taste and good taste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
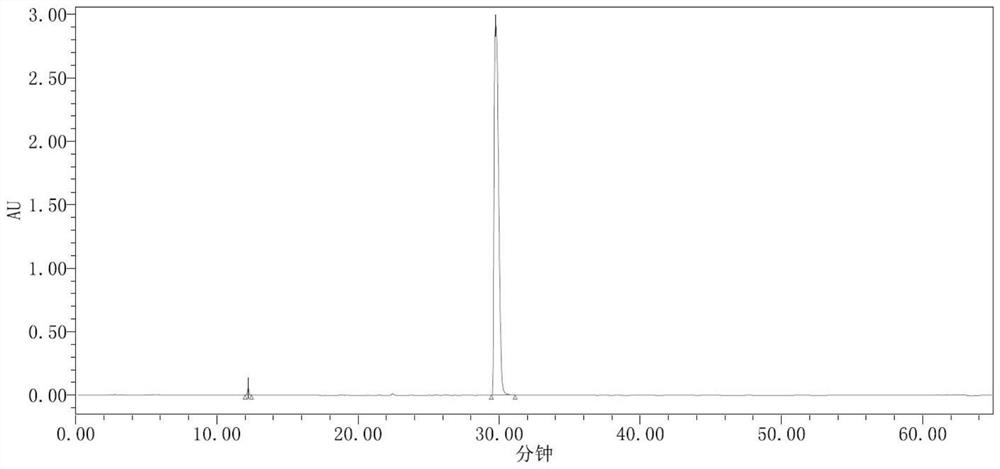
Image
Examples
Embodiment 1
[0027] A method for preparing a liquid formulation of Remdesivir solution for nebulizers. All utensils and containers need to be sterilized and carried out in Class D cleanliness (or not lower than Class D cleanliness) space.
[0028] In a 100ml beaker, add 1.5ml of absolute ethanol and 1.85g of sulfobutyl-β-cyclodextrin, first add 18ml of sterile water, stir to dissolve until clear, adjust the pH to 3.8 with 0.1M phosphoric acid, add and weigh Reid Xiwei 5.28g raw and auxiliary materials, ultrasonically dissolved until the system is clear, made up to 24ml with sterile pure water. Use a 0.22um membrane to filter the prepared medicinal solution above, dispense into vials (10ml bottles) of 8.0ml each, dispense into three vials, seal with a gland, and store in a refrigerator (2-8°C). That is, the liquid preparation of Remdesivir solution for nebulizers contains 110 mg of Remdesivir per tube, with a concentration of 13.75 mg / ml.
Embodiment 2-16
[0030] Configuration method and program are according to embodiment 1, press 3 bottles, every bottle 8ml configures, and its result is as follows
[0031]
[0032]
Embodiment 17
[0034] A method for preparing a liquid formulation of Remdesivir solution for nebulizers. All utensils and containers need to be sterilized and carried out in Class D cleanliness (or not lower than Class D cleanliness) space. In a 500ml beaker, add 20.0ml of absolute ethanol and 50.0g of sulfobutyl-β-cyclodextrin, first add 360ml of sterile water, stir and dissolve until clear, adjust the pH to 3.8 with 0.1M phosphoric acid, add and weigh Reid Xiwei 6.26g, ultrasonically dissolved until the system is clear, make up to 400ml with sterile pure water. Use a 0.22um membrane to filter the above prepared medicinal solution, dispense it into vials (10ml size), each bottle is dispensed with 8ml, and can be dispensed into 49 bottles, sealed with a gland, and stored in a refrigerator (2-8°C). That is, the liquid formulation of Remdesivir (Remdesivir) solution for nebulizers is 12.5 mg / ml, and each bottle contains 100 mg of Remdesivir (Remdesivir).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com