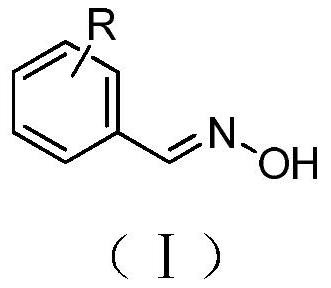
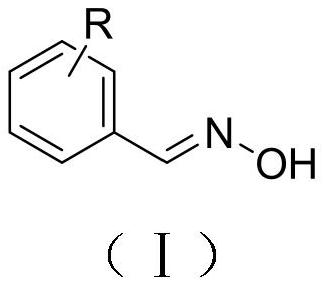
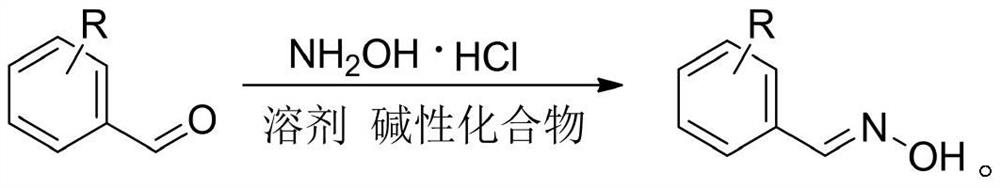
Microwave synthesis method of benzaldoxime compounds
A technology of benzaldehyde oxime and microwave synthesis, which is applied in oxime preparation, organic chemistry and other directions to achieve the effects of high yield, convenient operation and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The preferred of embodiment 1 experimental method
[0018] Dissolve the substituted benzaldehyde and the basic compound in the solvent, add hydroxylamine hydrochloride, stir at room temperature under the reaction calciner or react at room temperature in a microwave reactor, follow the reaction by TLC, filter after the reaction is completed, spin to dry the solvent, and use ethyl acetate Mix and extract with water 1:1, separate the organic phase and dry it with anhydrous sodium sulfate, filter and precipitate to obtain benzaldoxime derivatives, and optimize the reaction conditions, as shown in Table 1.
[0019] The comparison of productive rate under table 1 different operating techniques
[0020]
[0021]
[0022] As can be seen from Table 1, conventional method ethanol is made solvent normal temperature stirring 1-3 hour reaction is completed, and methanol is made solvent and needs room temperature stirring 3-4 hour reaction is completed, and ethanol is made solv...
Embodiment 2
[0023] The preparation of embodiment 2 benzaldoxime
[0024] Benzaldehyde (0.10g, 0.94mmol), hydroxylamine hydrochloride (0.08g, 1.16mmol), anhydrous sodium carbonate (0.12g, 1.17mmol) were dissolved in ethanol (3ml), placed in a microwave reactor and heated at 90°C, Reacted under the condition of 300W for 5 minutes, the reaction was completed, and the conversion rate detected by gas chromatography was 90.125%. The solvent was spin-dried, mixed with ethyl acetate (10ml) and water (10ml) for extraction, and the organic phase was separated and dried with anhydrous sodium sulfate. Filtration and precipitation to obtain the benzaldoxime represented by formula (A1).
[0025]
[0026] Benzaldehyde oxime: pale yellow oil, microwave yield 88.9%; 1 HNMR (CDCl 3 , 500MHz), δ: 8.93 (s, 1H, OH), 8.17 (s, 1H, CH), 7.56-7.24 (m, 5H, ph).
Embodiment 3
[0027] The preparation of embodiment 3 o-methylbenzaldehyde oxime
[0028] Dissolve o-tolualdehyde (0.10g, 0.83mmol), hydroxylamine hydrochloride (0.07g, 1.01mmol), anhydrous sodium carbonate (0.11g, 1.07mmol) in ethanol (3ml), place in a microwave reactor and React for 5 minutes under the conditions of 90°C and 300W. After the reaction is completed, the conversion rate detected by gas chromatography is 88.461%. The solvent is spin-dried, extracted with ethyl acetate (10ml) and water (10ml), and the organic phase is separated and washed with anhydrous sulfuric acid. Sodium drying, filtration, precipitation, the o-tolualdehyde oxime shown in formula (A2) is obtained.
[0029]
[0030] o-Tolualdehyde oxime: colorless oil, microwave yield 85.8%; 1 HNMR (CDCl 3 ,500MHz), δ:8.43(s,1H,CH),7.69-7.67(m,1H,ph),7.59(s,1H,OH),7.31-7.28(m,1H,ph),7.24-7.20( m,2H,ph),2.45(s,3H,CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com