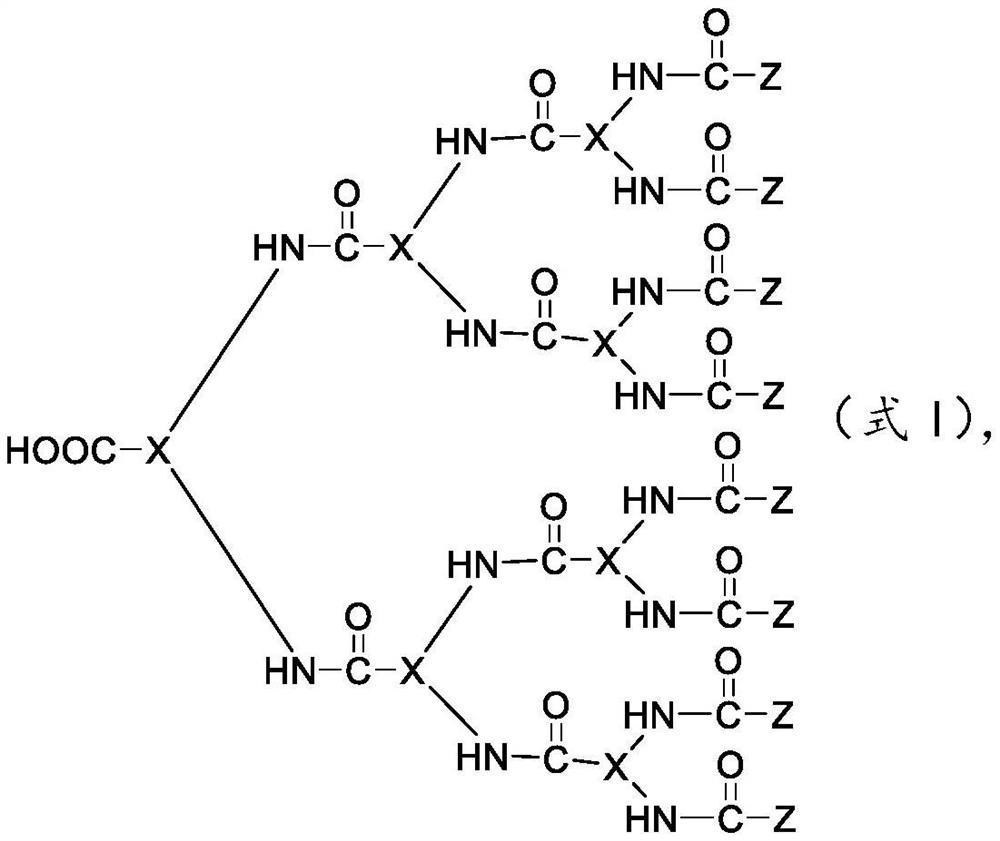
Pharmaceutical composition for preventing and/or treating coronavirus infection as well as preparation method and application thereof
A composition and drug technology, applied in the field of medicine, can solve problems such as undeveloped therapeutic drugs, achieve the effect of increasing the number and efficiency of action, strong specificity, and not easy to reject reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1 Preparation of Antigenic Peptides
[0063] Using software CLC Protein Workbench version 5.8 and Signal P5.0 software, SYFPE1TH1 remote prediction database, PREDEPP database to screen potential T cell single epitopes in SARS CoV-2 Env protein and cell membrane glycoproteins, and obtain potential HLA-A2 restricted Three single-epitope T cell antigen peptides: FLLVTLAIL, FLFLTWICL, SMWSFNPET.
[0064] The single-epitope T cell antigen peptide and the dendritic pluripotent T cell epitope peptide can be synthesized by conventional solid-phase synthesis. The amino acid sequence of each epitope peptide can also be synthesized by a peptide synthesis company.
Embodiment 2
[0065] Example 2 Preparation of dendritic cells loaded with pluripotent T cell epitope peptide
[0066] A method for preparing dendritic cells loaded with pluripotent T cell epitope peptides, comprising the steps of:
[0067] Human peripheral blood PBMCs were labeled with CD14+ magnetic beads, and CD14+ PBMCs were separated in a magnetic field; the sorted cells were added to DC culture bags with IL-4 (final concentration 30 ng / ml), GM-CSF (final Concentration is 60ng / ml) at 37°C, 5% CO 2 Co-cultivate in an incubator, the culture medium is PRMI1640 (containing 10% fetal bovine serum, 0.1ml penicillin-streptomycin), half of the culture medium is replaced every 3 days, and IL-4 and GM-CSF are added at the same time to induce dendrites generation of cells. After culturing for 10 days, cells that highly express HLA-I (77.31%), HLA-Dr (0.84%), CD80 (90.32%), CD86 (88.05%), and partially express CD40 (35.21%) were screened by flow cytometry , as the target DC cells.
[0068] Mi...
Embodiment 3
[0069] Example 3 Proliferative effect of DC cells loaded with pluripotent T cell epitope peptides on T cells
[0070] The WST-1 kit (ab155902, Abcam) was used to detect the stimulating effect of DC cells containing pluripotent T cell epitope peptides on the proliferation of T lymphocytes. The control group and the test group were respectively: DC cell group without antigenic peptide transformation, blank T cell group, DC cell group loaded with pluripotent T cell epitope peptide, and blank control group.
[0071] The specific measurement method is as follows:
[0072] Take a 96-well cell culture plate, add 0.1ml containing 2×10 5 RPMI1640 (containing 0.3% w / w PHA) culture medium of human peripheral blood T lymphocytes, at 37°C in 5% CO 2 After culturing for 2 hours, add 0.1ml (containing 2×10 4 cells) of DC cells loaded with pluripotent T cell epitope peptides, add 0.1ml (containing 2×10 4 cells) DC cells with untransformed antigen peptides, add 0.1ml (containing 2×10 cel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com