A kind of preparation method of high-purity sugammadex sodium
A technology of high-purity sugammadex sodium, applied in the field of preparation of high-purity sugammadex sodium, to achieve the effects of no genotoxicity, wide sources and reduction of metal ions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] Preparation of Crude Sugammadex Sodium
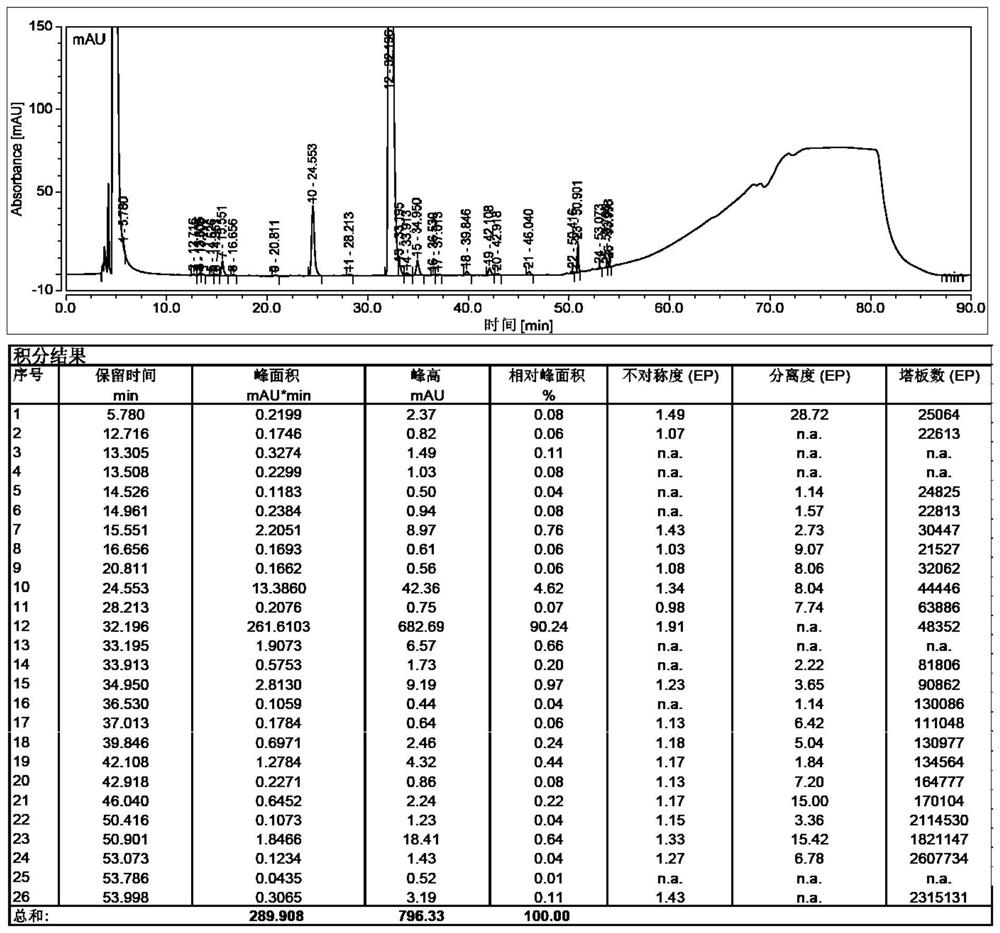
[0048] Put 3-mercaptopropionic acid (12.2mL, 140mol) into the reaction flask, add 450mL of N,N-dimethylformamide, add sodium hydride (12.3g, 308mol, 60% ), stirred at room temperature for 30 minutes after the addition, added dropwise γ-iodocyclodextrin (31.2g, 14mmol, dissolved in 450mL of N,N-dimethylformamide), after the dropwise addition was completed, the temperature was raised to 70 Degree, reaction 12h. After completion of the reaction, cool to room temperature, add 100mL of water, stir, distill under reduced pressure until the solvent remains 400mL, add 2L of ethanol, filter, collect the solid, and dry in vacuo to obtain 45g of off-white solid with a purity of 91.92%. See the test results figure 1 .
[0049] Sugammadex Sodium Injection Sterilization Color Inspection Experiment:
[0050] Take 1g of sugammadex sodium sample, under the protection of nitrogen filling, dissolve it in 8ml of water for injection, heat to diss...
Embodiment 1
[0054] Embodiment 1: Refining of sugammadex sodium
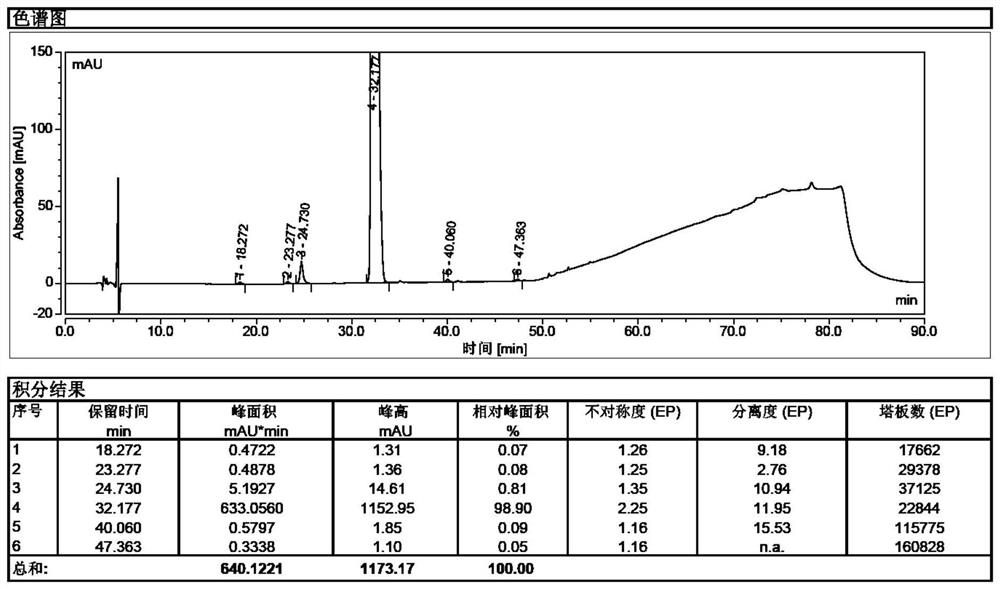
[0055] Take 100.0 g of sugammadex sodium crude product, dissolve it with 0.3 L of water until clear, add 2.0 g of inositol hexaphosphate (50% aqueous solution) under stirring, and raise the temperature to 80° C. under nitrogen protection, and add 0.8 L of After adding N,N-dimethylformamide, stir and cool down to room temperature, a large amount of white solid precipitates, filter with suction, and obtain 39.8g of pure sugammadex sodium with a purity of 99.8%, see figure 2 .
[0056] Of course, stirring and cooling can also be carried out to -20-30 degrees Celsius.
Embodiment 2
[0057] Embodiment 2: Refining of sugammadex sodium
[0058] Take 100 g of sugammadex sodium crude product, dissolve it with 1 L of water until clear, add 1 g of inositol diphosphate under stirring, raise the temperature to 70°C under nitrogen protection, and add 6 L of acetonitrile to the solution. After the addition was completed, the temperature was lowered to room temperature with stirring, and a large amount of white solids were precipitated. After suction filtration, 32.3 g of pure sugammadex sodium was obtained, with a purity of 99.5%.
[0059] The protective gas can also be one selected from nitrogen, argon, helium, and carbon dioxide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com