Application of pirfenidone to preparation of medicine for preventing and treating rheumatoid arthritis
A pirfenidone and rheumatoid technology, applied in the field of drugs for preventing and treating rheumatoid arthritis, can solve the problems of ineffective prevention, large side effects, single function, etc., and achieves inhibition of angiogenesis, inhibition of inflammatory response, and obvious inhibition effect of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
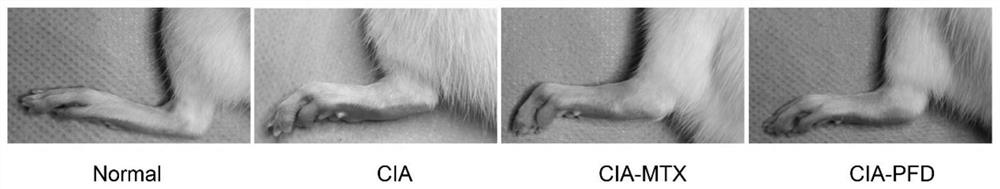
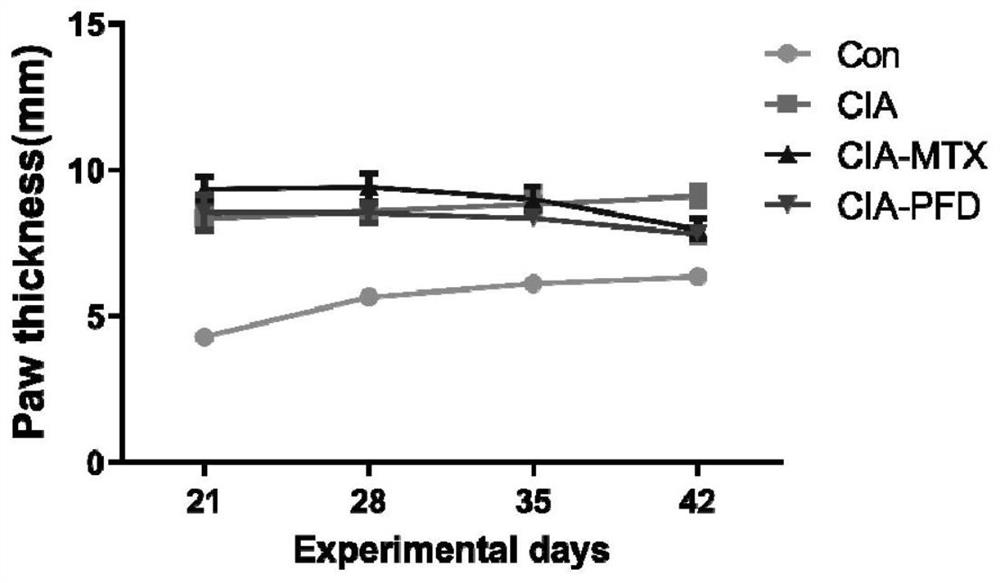
[0109] Measurement and analysis of the improvement effect of PFD on foot swelling in CIA rats
[0110] Twenty-four male Lewis rats (8 weeks old) were raised in a pathogen-free environment. The 24 rats were randomly divided into 4 groups (6 rats / group): normal group (Normal, first group) and 3 CIA model groups (second to fourth groups). First, establish the CIA rat model: mix the same amount of bovine type II collagen and incomplete adjuvant with a glass syringe, inject 200 μl of the suspension subcutaneously at a place 2 cm from the root of the tail of the rat, and after 7 days, follow the above method, each Inject 100 μl of the suspension at the base of the tail of the mouse to strengthen the immune response, and after 14 days, it is confirmed that the modeling is successful. After the CIA model was successfully established, the mice in the second group were given carboxymethylcellulose sodium suspension, 10 ml / kg / day (CIA), and the mice in the third group were given methotr...
experiment example 2
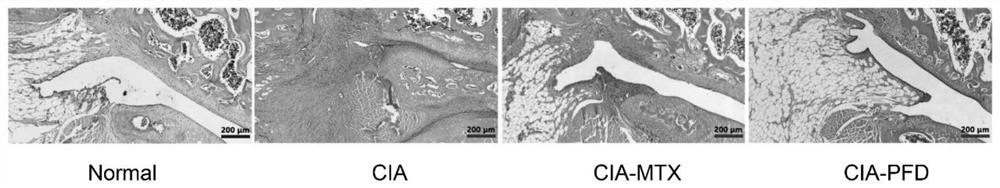
[0113] Using hematoxylin and eosin staining to detect the effect of PFD on synovial inflammation of CIA rats
Embodiment 1
[0114] After the rats in Example 1 were euthanized, the right knee joint was fixed with 4% paraformaldehyde, decalcified with 10% EDTA for 40 days, followed by routine dehydration, paraffin embedding, and 5-μm sectioning. Then hematoxylin and eosin (H&E) staining was performed on the sections: the paraffin sections of knee joints were routinely dewaxed and rehydrated with xylene and gradient alcohol, stained with hematoxylin staining solution for 7 minutes, and the excess staining solution was rinsed before use. Stain with Yin Hong for 1 minute, then use graded alcohol to dehydrate, clear with xylene, then mount with neutral gum, and finally take pictures of the sections for analysis. The scoring criteria for joint synovial inflammation are: 0 = no inflammation, 1 = slightly thickened synovial lining or inflammatory cell infiltration, 2 = slightly thickened synovial lining with inflammatory cell infiltration, 3 = synovial lining Thickened layer, synovial space infiltration of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com