Impurity compound of nitrocaphane, and preparation method and application of impurity compound
A compound, the technology of nitro mustard, which is applied in the field of medicine, can solve the problems of unknown impurity structure and content uncertainty, unfavorable control or improvement of the quality standard of nitro mustard, and no preparation, so as to improve the safety of clinical medication and the preparation method is simple and efficient , the effect of improving quality standards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
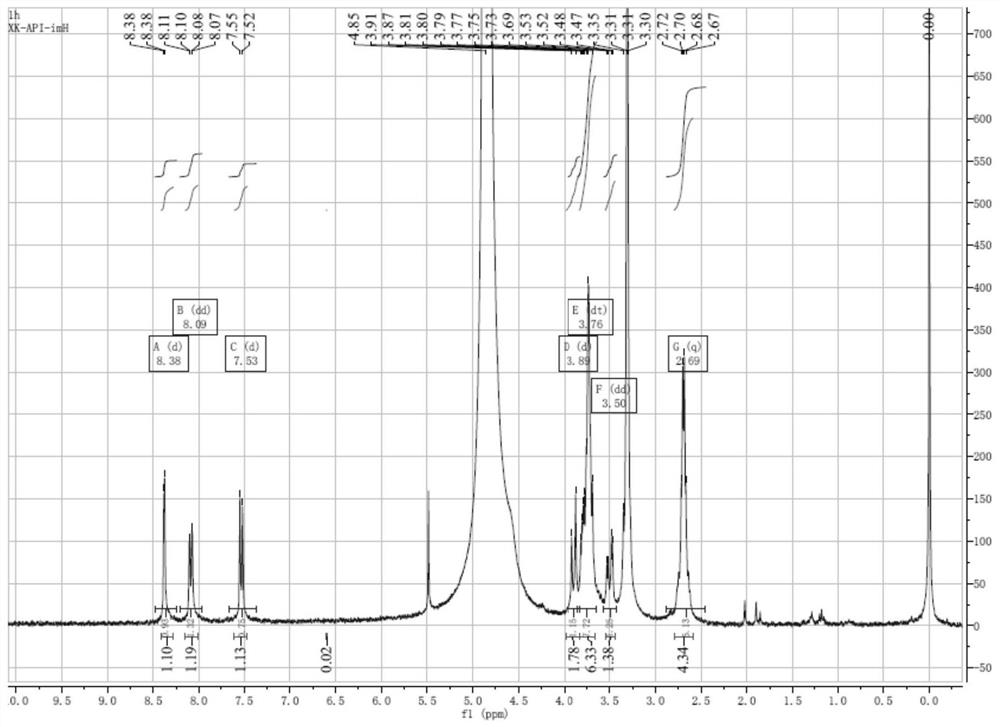
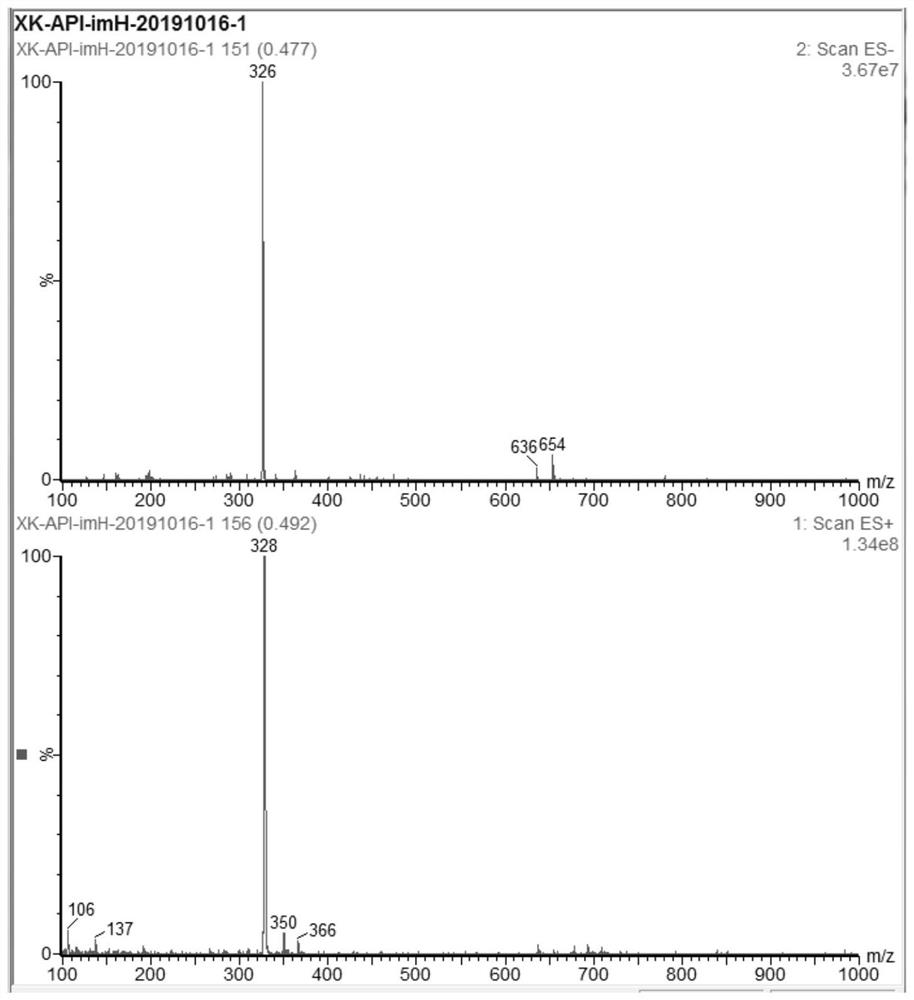
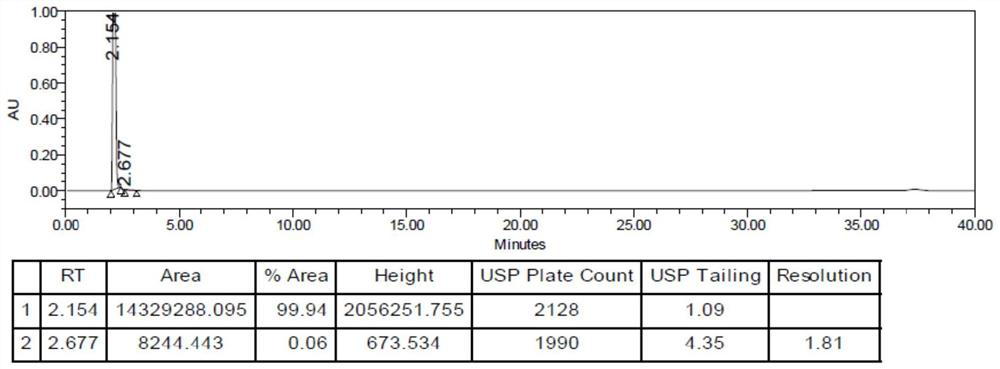
Image
Examples
preparation example Construction
[0061] The embodiment of the present invention also provides a preparation method of the above-mentioned impurity compound of nitro mustard, comprising:
[0062] Utilize compound I and the compound that contains R group to carry out condensation reaction to obtain intermediate, wherein, the structural formula of compound I and described intermediate is shown as follows successively:
[0063] as well as Wherein, X represents a halogen, for example, any one of chlorine, bromine and iodine that can be used, and R is or R 1 and R 2 Each independently is an alkyl group, further is a C1-C8 alkyl group, preferably any one of methyl, ethyl, propyl, n-butyl, isobutyl, tert-butyl and pentyl; R 3 It is an amino protecting group, and the amino protecting group is a group that protects NH to prevent it from reacting, and the amino protecting group is R 3 The amino protecting group is Boc, Tfa, phenylacetyl or any of the.
[0064] It should be noted that although R in the examp...
Embodiment 1
[0079] This embodiment provides a compound shown in the following structural formula, namely the preparation process of compound 1:
[0080]
[0081] S1, condensation reaction
[0082] Weigh 10.00g of compound I, 4.72g of compound II, and 100ml of ethyl acetate into a 250ml three-neck flask, stir and heat up to 80°C, and reflux for 6h. TLC detected that the reaction was complete, and post-treatment was adding 30 ml of water to wash twice, and phase separation to obtain an organic phase. Add 100ml of n-heptane to the organic phase at room temperature and stir for crystallization for 2h. A large amount of off-white solid was precipitated, and the intermediate was obtained by suction filtration, 9.88 g of white solid, yield 93.73%.
[0083] The structural formulas of compound I, compound II and intermediates are as follows:
[0084] as well as
[0085] S2, decarboxylation reaction
[0086] Weigh 5.00g of the intermediate, add 20ml of concentrated hydrochloric acid (mo...
Embodiment 2- Embodiment 3
[0088] Embodiment 2-Example 3 provides a kind of preparation process of compound 1, its operation and the preparation process operation of compound 1 provided in embodiment 1, the difference is that the operating conditions are different, as follows:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com