Mouse bone marrow dendritic cell induction and purification method
A dendritic cell and purification method technology, applied in the field of mouse bone marrow dendritic cell induction and purification, can solve the problems of small number, complicated operation, long dendritic cell induction period, etc., and achieve the effect of cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A method for inducing and purifying mouse bone marrow dendritic cells, comprising the following steps:
[0029] Extraction of S1 mouse bone marrow cells:
[0030] S101. Select 4-week-old mice to be killed, soak them in 75% alcohol and put them into an ultra-clean table. After 3-5 minutes, take them out and put them into a 10cm-diameter sterile petri dish;
[0031] S102. Carefully pinch the abdominal skin of the iliac fossa of the mouse with sterile tweezers, carefully cut the skin with ophthalmic scissors, remove the skin from the thigh to the ankle joint from top to bottom, cut at the ankle and hip joint, and free the mouse two lower limbs;
[0032] S103. Peel off the muscles of the knee joint, and split them at the junction of the femur and tibia against the direction of joint bending, and the tibial plateau can be seen; remove the appendages of the distal end of the femur, use gauze to remove the muscles attached to the bone, and put it in a 75% alcohol-filled conta...
Embodiment 2
[0051] In step S104, the epiphysis is not reserved, and other steps are the same as in embodiment 1.
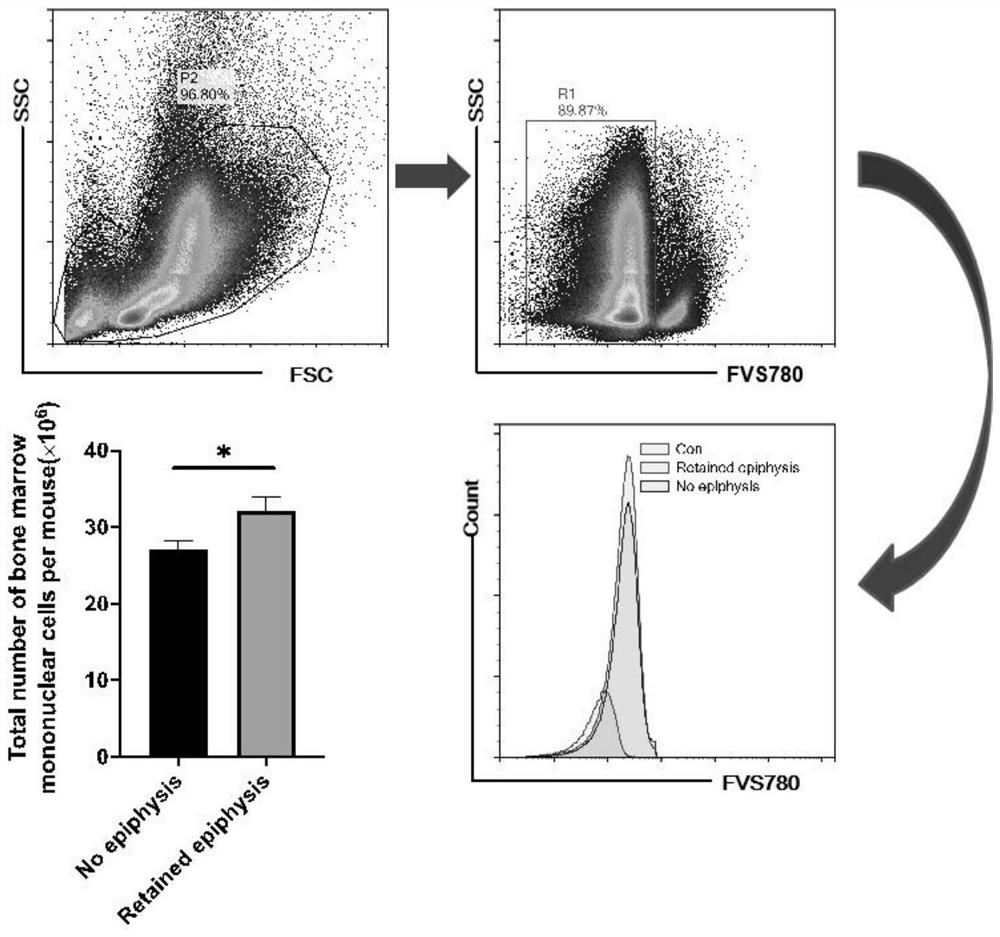
[0052] like figure 1 As shown, incubate with FVS-780 dead and alive dye for 15 minutes, and perform flow cytometry after elution with PBS. The ratio of living cells R1 accounts for more than 90% of the total cells in the P2 gate; count the number of cells in the R1 gate and calculate the total number of cells, and keep the epiphysis The number of cells in the non-retained group increased by 18%.
Embodiment 3
[0054] Traditional method group [Traditional group (T)]:
[0055] ① On day 0, according to the number of viable bone marrow cells, adjust the cell concentration to 4×105 cells / mL with 10% RPMI-1640 medium containing GM-CSF, IL-4 (the final concentration is 40 ng / mL), Take 10mL of cell suspension and transfer it into a 10cm diameter petri dish (three replicate wells), and place it at 37°C with 5% CO 2 Cultivated in an incubator;
[0056] ② On the third day, completely remove the old culture medium containing cells in the dish, and add 10 mL of the above fresh culture medium containing the same inducer in ①;
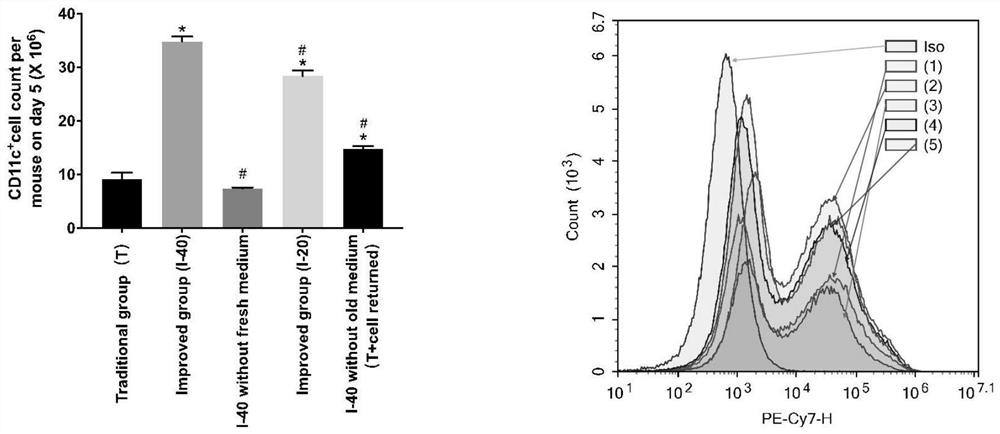
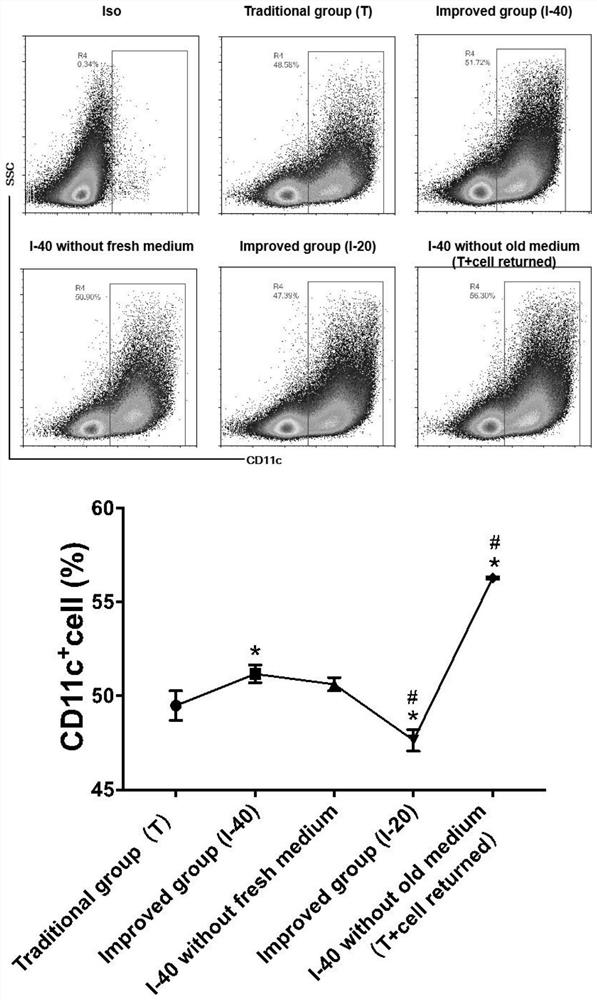
[0057] ③ On day 5, take the cell suspension in the culture dish, centrifuge at 300G for 10 minutes, wash with PBS three times, block with FCR at 4°C for 15 minutes, add CD11c flow cytometry antibody and incubate at 4°C for 30 minutes, wash with PBS three times, and measure the number of cells by flow cytometry and purity, the total amount of dendritic cells was calculate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com