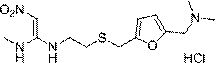
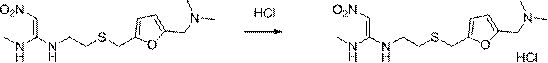Preparation method of ranitidine hydrochloride with low NDMA content
A technology of ranitidine hydrochloride and ranitidine base, which is applied in the field of preparation of ranitidine hydrochloride with low NDMA content, can solve the problems of high processing cost, increased N-dimethylnitrosamine impurity content, and salt formation Problems such as poor properties and color of the final product, to achieve the effect of simple operation, reduced processing cost, and good product properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] A preparation method of ranitidine hydrochloride with low NDMA content, comprising the following steps:
[0024] S1. Add ranitidine base to ethanol and stir until completely dissolved; the mass ratio of ranitidine base to ethanol is 1:3-5;
[0025] S2. Cool the solution to -5-5°C, add hydrochloric acid aqueous solution, adjust the pH to 4.5-6.5, and stir evenly; the concentration of the aqueous hydrochloric acid solution is 20%-38%, preferably 20%-25%;
[0026] S3, adding seed crystals, heat-preserving, stirring and crystallizing for 3-5 hours; the heat-preserving crystallization temperature is -5-5°C, and the stirring speed is 100-300rpm;
[0027] S4. The crystals are filtered, washed, drained and dried to obtain off-white crystalline solid ranitidine hydrochloride. Absolute ethanol is used for washing; vacuum drying is used, the temperature is controlled at 65-75°C, the time is 3-5h, and the vacuum degree is 0.09Mpa.
[0028]
Embodiment 1
[0030] Add 50g of ranitidine base to a 500ml reaction bottle, then add 300ml of absolute ethanol, stir and dissolve at room temperature; cool down to about -5°C, then slowly add 20% hydrochloric acid aqueous solution to the reaction bottle to adjust the pH value between 4.5-5.5 After the dropwise addition, add seed crystals, keep warm at -5°C for 5 hours, and stir and crystallize at 100rpm; the precipitated solid is filtered out with suction, and the filter cake is rinsed with 50ml of absolute ethanol; the filter cake is vacuum-dried at 65°C for 5 hours under reduced pressure to obtain Off-white crystalline solid 44.79g, molar yield 80.28%. HPLC content 99.6%, residual ethanol 0.24%, NDMA content 0.018PPM.
Embodiment 2
[0032] Add 120g of ranitidine base to a 1L reaction bottle, then add 600ml of absolute ethanol, stir and dissolve at room temperature; cool down to about 0°C, then slowly add 23% hydrochloric acid aqueous solution to the reaction bottle to adjust the pH value between 5.0-6.0 After the dropwise addition, add seed crystals, insulate at about 0°C and stir for crystallization for 4 hours, and the rotating speed is 200rpm; the precipitated solid is filtered out by suction, and the filter cake is rinsed with 100ml of absolute ethanol; the filter cake is vacuum-dried at 70°C for 4 hours under reduced pressure to obtain a White crystalline solid 117.42g, molar yield 83.67%. HPLC content 99.7%, residual ethanol 0.31%, NDMA content 0.036PPM.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com