Boron-containing polymer solid electrolyte and application thereof
A technology of solid electrolyte and composite electrolyte, which is applied in the direction of composite electrolytes, circuits, electrical components, etc., which can solve the problems of difficulty in meeting the application requirements of high-voltage electrode systems, low ionic conductivity, and low electrochemical window, and achieve excellent lithium ion conduction performance, reducing segmental crystallinity, and improving flexibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Such as figure 1 As shown, this embodiment provides a method for preparing a boron-containing polymer solid state electrolyte, which includes the following steps:
[0042]Weigh 1.236g of boric acid, 4.4g of PEG200, and 4.9g of mPEG350 respectively, add the three into the flask, stir at room temperature for 15min, vacuumize and keep it; after the degassing of the mixture is complete, heat and stir at 160°C for 4h, collect the product, Transfer to a glove box, weigh 1.0047g LiTFSI and mix it with the product, heat and dissolve at 150°C to obtain a boron-containing polymer solid electrolyte M1.
[0043] The method for preparing a polymer solid electrolyte membrane by using the above-mentioned boron-containing polymer solid electrolyte M1 is as follows:
[0044] Weigh 2g of boron-containing polymer solid electrolyte M1 and 4g of dimethyl sulfoxide, mix, heat and stir at 40°C for 2h to obtain a solution, weigh 4g of the solution and pour it onto the surface of a clean glass...
Embodiment 2
[0048] This embodiment provides a method for preparing a boron-containing polymer solid electrolyte, which includes the following steps:
[0049] Weigh 1.854g of boric acid, 7.5g of PEG200, and 4.2g of mPEG350 respectively, add the three into the flask and stir at room temperature for 15min, vacuumize and keep it. After the mixture is completely defoamed, heat and stir at 160°C for 4h, collect the product, and transfer to In the glove box, weigh 0.8612g LiTFSI and mix with the product, heat and dissolve at 150°C to obtain boron-containing polymer solid electrolyte M2. figure 1 Schematic diagram of the structure of the polymer electrolyte prepared for Example 2 of the present invention, specifically, the polymer electrolyte is an elastomer clamped by metal tweezers.
[0050] Using the boron-containing polymer solid electrolyte M2, adopt the same electrolyte membrane preparation method and button battery preparation method as in Example 1, and carry out EIS test and CV test, and...
Embodiment 3
[0052] This embodiment provides a method for preparing a boron-containing polymer solid electrolyte, which includes the following steps:
[0053] Weigh 1.236g of boric acid, 9.6g of PPG500, and 3.5g of mPEG350 respectively, add the three into the flask and stir at room temperature for 15min, vacuumize and keep it, after the mixture is completely defoamed, heat at 180°C and stir at 100rpm for 6h, collect the product, transfer Put it into a glove box, weigh 0.7177g LiTFSI and mix it with the product, heat and dissolve at 160°C to obtain the boron-containing polymer solid electrolyte M3.
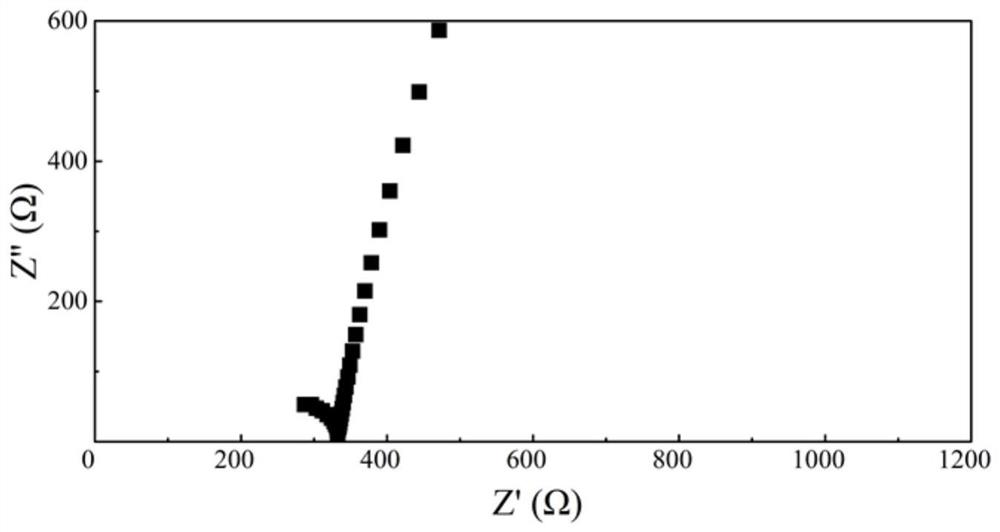
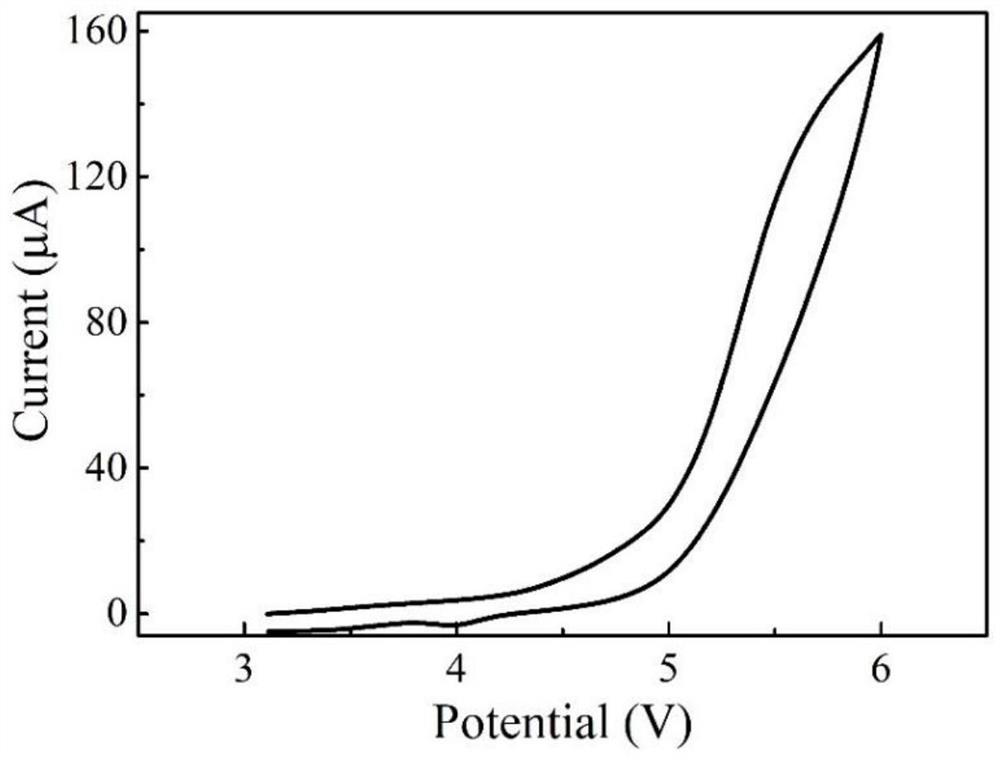
[0054] Using the boron-containing polymer solid electrolyte M3, adopt the same electrolyte membrane preparation method and button battery preparation method as in Example 1, and carry out EIS test and CV test, and the measured ion conductivity of the boron-containing polymer solid electrolyte M3 is 0.37mS / cm, the electrochemical window is 4.5V. figure 2 The EIS test curve of the polymer elec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com