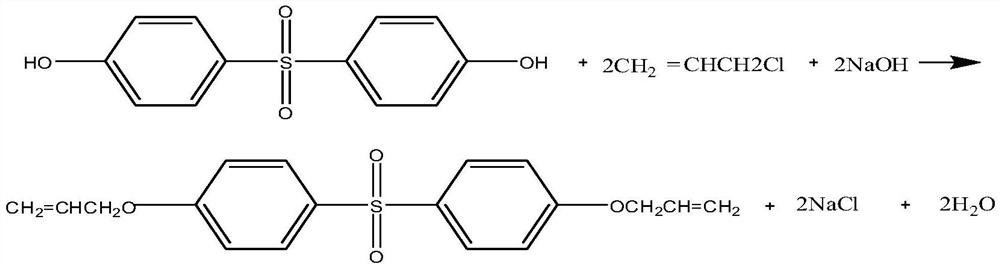
The preparation method of diallyl bisphenol S ether
A diallyl bisphenol and solvent technology, which is applied in the field of preparation of diallyl bisphenol S ether, can solve the problems of unavoidable, expensive catalysts in the subsequent process of wastewater treatment, etc., and achieves increased usage and improved disposable Conversion rate, the effect of reducing product cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
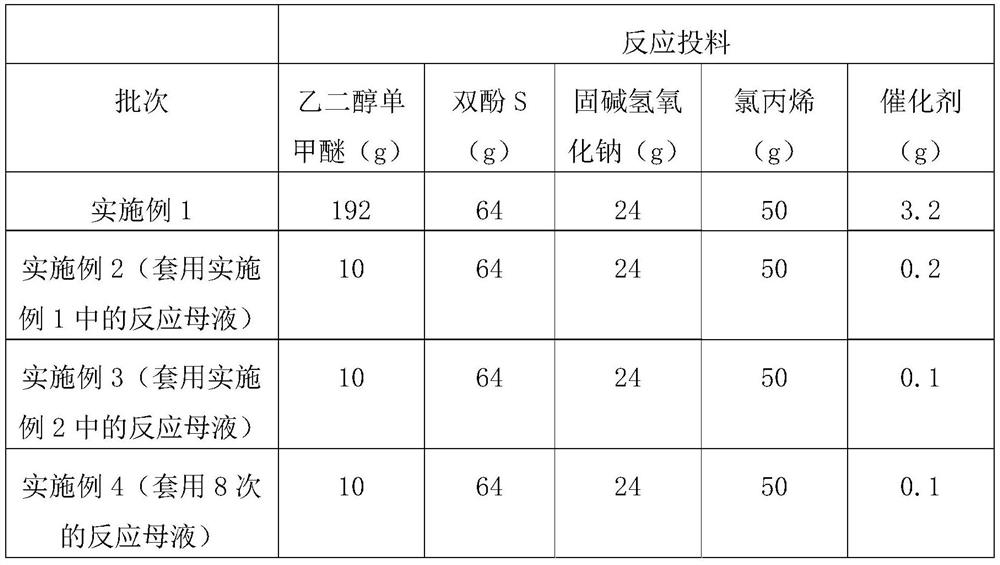
[0025] In a three-neck flask with stirring, add 192 grams of diethylene glycol monomethyl ether, 24 grams of sodium hydroxide, stir for about 10 minutes, add 64 grams of bisphenol S under stirring conditions, then add 3.2 grams of catalyst, and stir for 10 minutes Finally, add 50 grams of allyl chloride, replace the environment in the kettle with nitrogen, seal the reactor, adjust the reaction temperature to 60°C, then gradually adjust the reaction temperature to 100°C, maintain the reaction temperature for 5 hours, cool to room temperature and filter, and leave the reaction mother liquor apply.
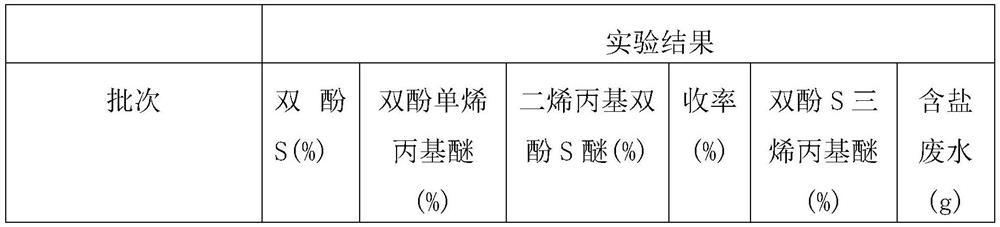
[0026] After filtering, add 250 grams of toluene to the obtained filter cake, heat and dissolve the product and then heat filter, wash the filter cake with toluene to obtain sodium chloride salt with a purity of 99%, then concentrate the mother liquor, crystallize, filter, and dry Finally, 69 grams of diallyl bisphenol S ether was obtained, with a content of 99.6% and a yield of 82%....
Embodiment 2
[0028] In the three-neck flask with stirring, add the reaction mother liquor obtained by filtering in Example 1, add 10 grams of solvent, 24 grams of sodium hydroxide, stir for about 10 minutes, add 64 grams of bisphenol S under stirring conditions, and then add the catalyst 0.2 grams, after stirring for 10 minutes, add 50 grams of allyl chloride, replace the environment in the kettle with nitrogen, seal the reactor, adjust the reaction temperature to 60°C, then gradually adjust the reaction temperature to 100°C, maintain the reaction temperature for 5 hours, and then cool down to Filter at room temperature, and keep the mother liquor for future use.
[0029] Sodium chloride and bisphenol S diallyl ether were separated from the filter cake according to the method used in Example 1. Test result: diallyl bisphenol S ether 77.7 grams, content 99.7%, yield 92%.
Embodiment 3
[0031] In the there-necked flask with stirring, add the reaction mother liquor obtained by filtering in Example 2, add 10 grams of solvent, 24 grams of sodium hydroxide, stir for about 10 minutes, add 64 grams of bisphenol S under stirring conditions, and then add the catalyst 0.1 g, after stirring for 10 minutes, add 50 g of allyl chloride, replace the environment in the kettle with nitrogen, seal the reactor, adjust the reaction temperature to 60°C, and then gradually adjust the reaction temperature to 100°C, maintain the temperature for 5 hours, cool down to room temperature and filter .
[0032] Sodium chloride and bisphenol S diallyl ether were separated from the filter cake according to the method used in Example 1. Test result: Diallyl bisphenol S ether 78.6 grams, content 99.6%, yield 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com