Double-electron compound flow battery system based on salt cavern
An electronic compound and liquid flow battery technology, applied in fuel cells, electrochemical generators, regenerative fuel cells, etc., can solve the problems of limited solubility of active materials, prone to water electrolysis side reactions, and easy cross-contamination of electrolytes, etc., to achieve Ease of serialization, low production cost, and less production equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
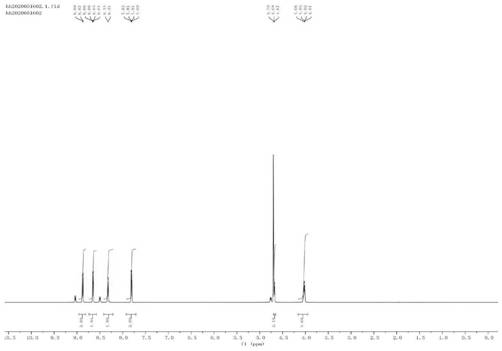
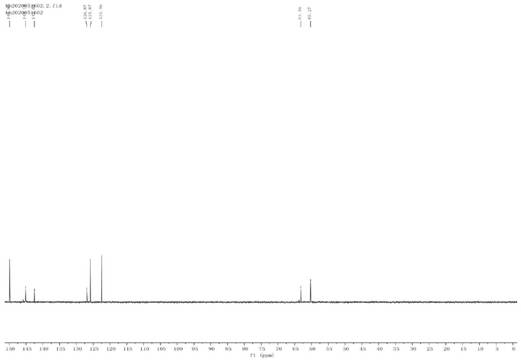
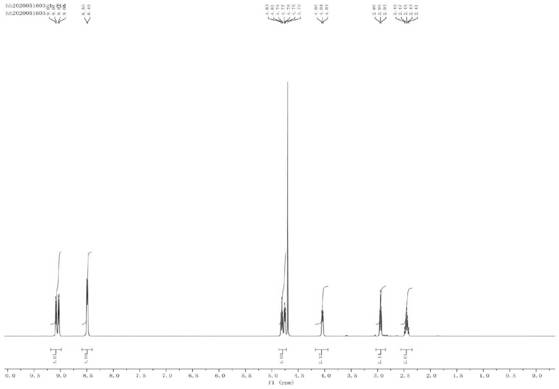
Image
Examples
Embodiment 1
[0067] Synthesis of TEMPO-4-sulfonic acid sodium salt
[0068] Add 5g (29.03mmol) 4-OH-TEMPO and 20mL dichloromethane solvent into the three-necked flask, after the 4-OH-TEMPO dissolves, add 2mL chlorosulfonic acid dropwise at room temperature, and continue stirring at room temperature for 20 minutes after the addition. After the reaction, saturated sodium bicarbonate solution was added dropwise to adjust the pH of the resulting reaction solution to 7, and then extracted twice with ethyl acetate to remove unreacted 4-OH-TEMPO. The solvent was spin-dried under reduced pressure to obtain a mixture containing TEMPO-4-sulfonic acid sodium salt and sodium sulfonate. The TEMPO-4-sulfonic acid sodium salt in the mixed solid was extracted with absolute ethanol, and the solvent was removed under reduced pressure to obtain a yellow solid. NMR characterization was not carried out due to free radicals contained in the molecule.
Embodiment 2
[0070] Synthesis of 1-(2-hydroxy-ethyl)-[4,4'-bipyridine]-1-onium bromide
[0071] Add 3.436g (22mmol) 4,4'-bipyridine and 50mL acetonitrile solvent into the three-necked flask, add 2.631g (20mmol95%) 2-bromoethanol after dissolving 4,4'-bipyridine, heat up to 90°C and reflux After stirring the reaction, an off-white precipitate gradually appeared after 8 hours, and the reaction was continued for 40 hours until the reaction was complete. The resulting reaction solution was cooled and suction-filtered, washed 3 times, and finally vacuum-dried to obtain 5.628 g of off-white powdery solid particles (named 1-(2-hydroxy-ethyl)-[4,4'-bipyridine]-1 -onium bromide, yield 91%)).
Embodiment 3
[0073] Synthesis of 1-(2-hydroxy-ethyl)-1'-(3-sulfo-propyl)-[4,4'-bipyridine]-1,1'-dibromide
[0074] Add 5.904g (21mmol) 1-(2-hydroxyl-ethyl)-[4,4'-bipyridine]-1-bromide, 2.467g (20mmol, 99%) 1,3- Propane sultone and 50mL acetonitrile solvent were heated to 90°C under reflux and stirred for reaction. After 4 hours, the off-white precipitate began to change into a light yellow precipitate, and the reaction was continued for 20 hours until complete reaction. The resulting reaction solution was cooled and suction-filtered, washed 3 times, and finally vacuum-dried to obtain 7.410 g of light yellow powdery solid particles (named 1-(2-hydroxyl-ethyl)-1'-(3-sulfonic acid group- Propyl)-[4,4'-bipyridine]-1,1'-onium bromide, yield 87.5%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com