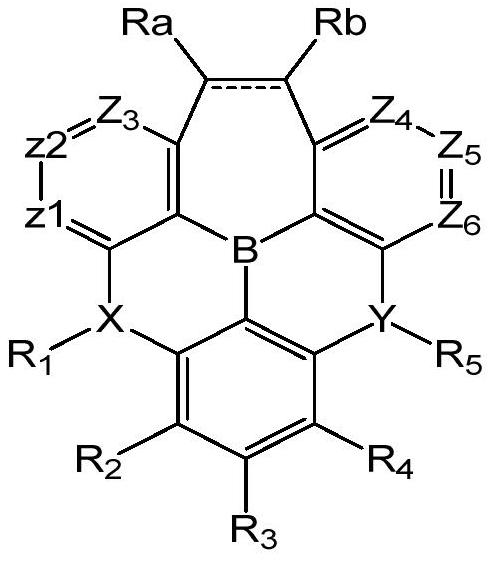
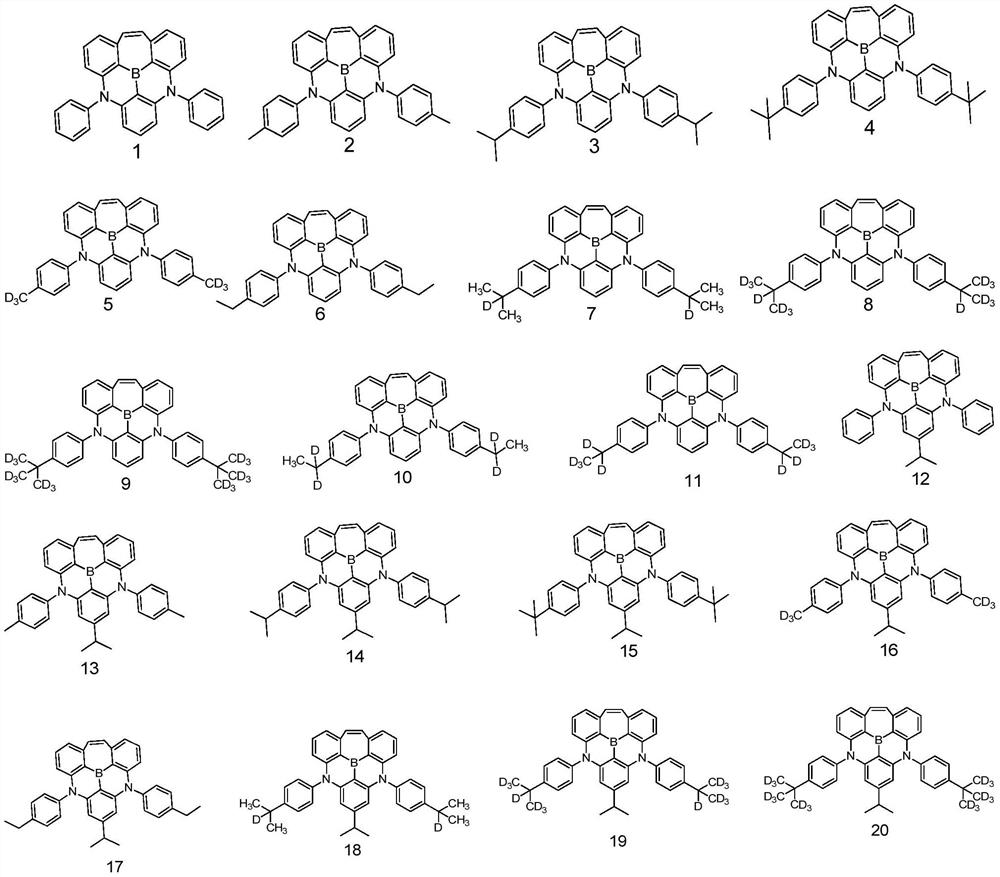
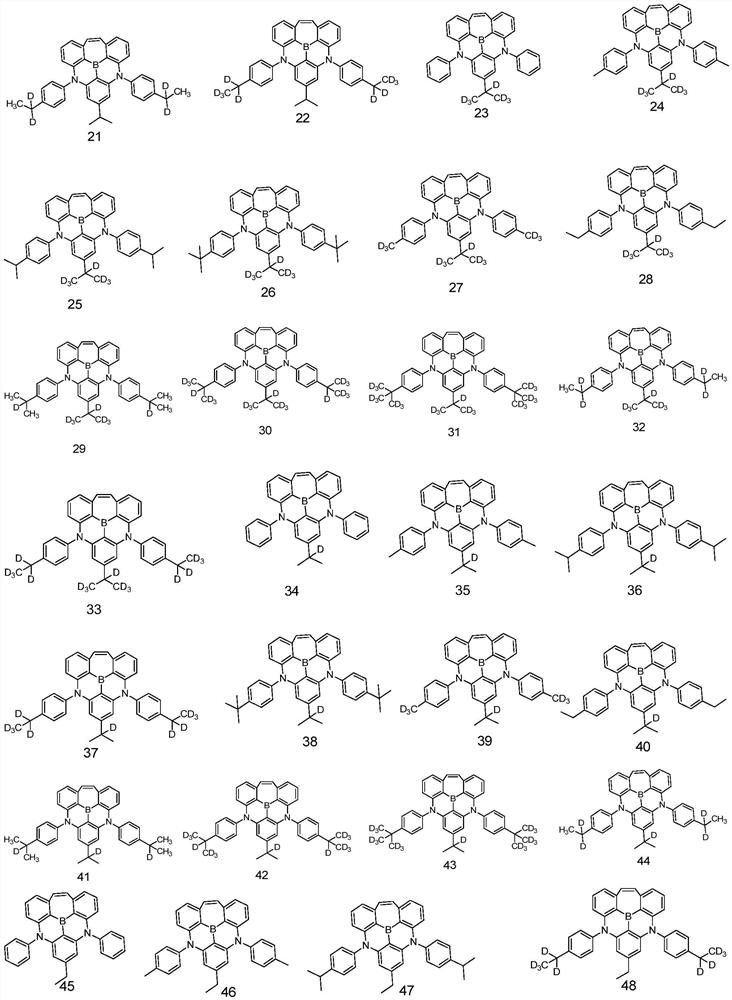
High-efficiency dark blue fluorescence doped material and OLED organic electroluminescent device
A doping material, dark blue technology, applied in the field of high-efficiency dark blue fluorescent doping materials and OLED organic electroluminescent devices, can solve the problems of insufficient development of organic electroluminescent materials and backward panel manufacturing enterprises, etc., to achieve high efficiency and stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]
[0045] 1 is synthesized as follows:
[0046] (1)
[0047]Under nitrogen protection, compound 1 (100g, 267.83g / mol, 373.4mmol), compound 2 (2.1eq, 93g / mol, 784.14mmol, 72.9g), sodium tert-butoxide (2.1eq, 96.1g / mol, 784.14 mmol, 75.36g), Pd2(dba)3 (palladium dibenzylideneacetonate, 5%eq, 915.72g / mol, 18.67mmol, 17.09g), tri-tert-butylphosphine (5%eq, 202.317g / mol , 18.67mmol, 3.78g), toluene (1000g, 10 times the mass of compound 1) were added to the reaction flask, and after the addition was completed, the temperature was raised to reflux for 12 hours. After the HPLC detection, the reaction was completed, and after cooling down to room temperature, water was added and stirred for 15 minutes, and then filtered to obtain The filtrate, after the filtrate is separated, the organic phase is obtained. The organic phase is dried with anhydrous magnesium sulfate and passed through a silica gel funnel to obtain the secondary filtrate. After purification, high-purity comp...
Embodiment 2
[0053]
[0054] The synthesis method of 8 is as follows:
[0055] (1)
[0056] Under nitrogen protection, compound 1 (10g, 267.83g / mol, 37.34mmol), compound 6 (1.1eq, 142.15g / mol, 41.07mmol, 5.84g), sodium tert-butoxide (1.1eq, 96.1g / mol, 41.07mmol, 3.95g), Pd2(dba)3 (5%eq, 915.72g / mol, 1.87mmol, 1.71g), tri-tert-butylphosphine (5%eq, 202.317g / mol, 1.87mmol, 0.38g) 1. Toluene (100g, 10 times the quality of compound 1) was added to the reaction flask, and after the addition was completed, the temperature was raised to reflux for 12 hours. After the HPLC detection reaction was completed, it was cooled to room temperature, then added water and stirred for 15 minutes to obtain the filtrate. The filtrate was obtained after liquid separation. The organic phase, the organic phase is dried with anhydrous magnesium sulfate and passed through a silica gel funnel to obtain the secondary filtrate. After rotary evaporation, an appropriate amount of dichloromethane is added to complet...
Embodiment 3
[0064]
[0065] The synthetic method of 13 is as follows:
[0066] (1)
[0067] Under nitrogen protection, compound 11 (100g, 309.88g / mol, 322.71mmol), compound 12 (2.1eq, 107.07g / mol, 677.69mmol, 72.56g), sodium tert-butoxide (2.1eq, 96.1g / mol, 677.69mmol, 65.13g), Pd2(dba)3 (5%eq, 915.72g / mol, 16.14mmol, 14.78g), tri-tert-butylphosphine (5%eq, 202.317g / mol, 16.14mmol, 3.27g) 1. Toluene (1000g, 10 times the quality of compound 11) was added to the reaction flask, and after the addition was completed, the temperature was raised to reflux for 12 hours. After the HPLC detection reaction was completed, after the reaction was completed, it was cooled to room temperature and then added with water and stirred for 15 minutes to obtain the filtrate. The filtrate was obtained after liquid separation. The organic phase, the organic phase is dried with anhydrous magnesium sulfate and passed through a silica gel funnel to obtain the secondary filtrate. After rotary evaporation, an a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com