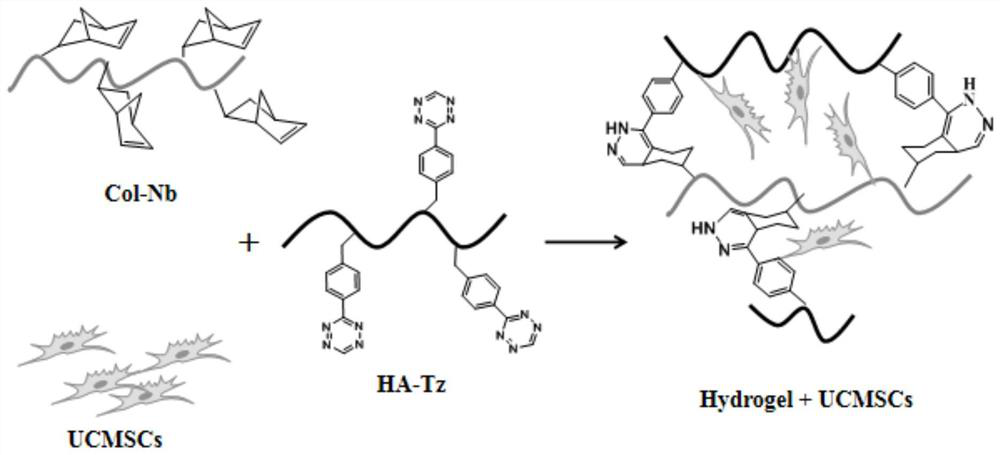
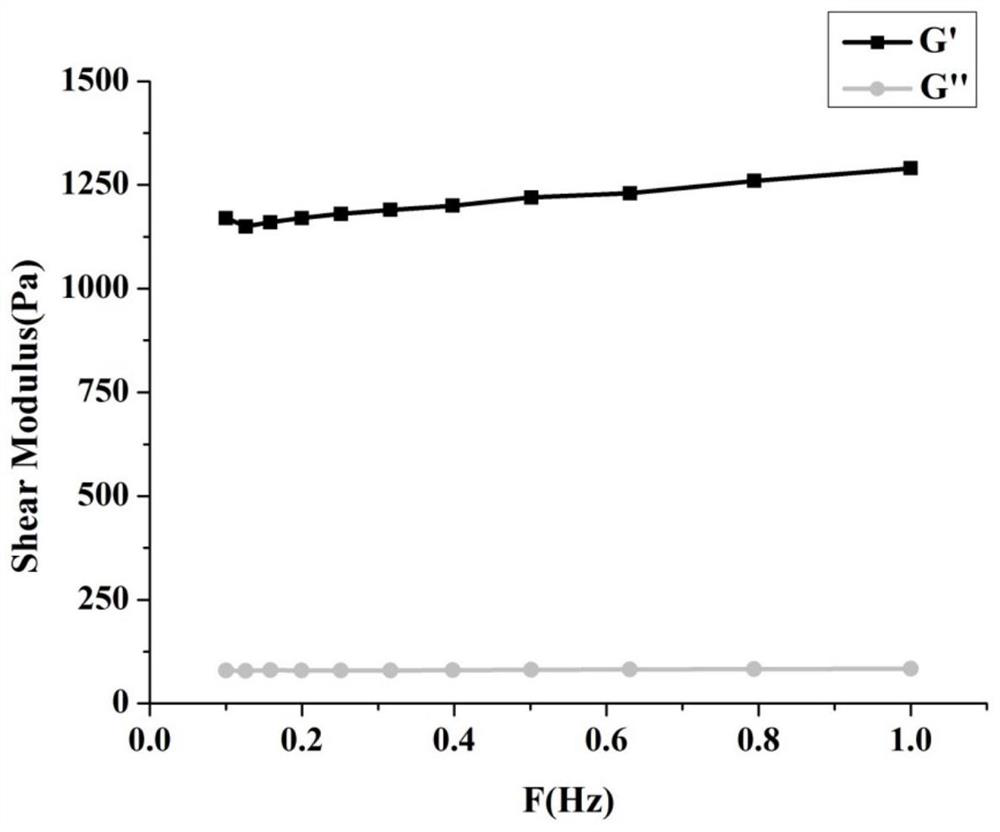
Rapidly curable hydrogel based on collagen and hyaluronic acid, its preparation method and application
A technology of hyaluronic acid and hydrogel, applied in prosthesis, medical science and other directions, can solve the problems of difficult chemical modification, low biocompatibility, incomplete degradation, etc., and achieve low toxicity, simple preparation method, excellent biological Compatibility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0071] In some embodiments, the preparation method includes: mixing hyaluronic acid, 3-(4-benzylamino)-1,2,4,5-tetrazine, a condensing agent and a first solvent to form a first mixed system A step of obtaining tetrazine-modified hyaluronic acid by amidation reaction; wherein, the reaction temperature is controlled at 15-30° C., and the reaction time is controlled at 10-20 hours.
[0072] Preferably, the condensing agent includes benzotriazol-1-yloxy tris(dimethylamino)phosphonium hexafluorophosphate and N,N-diisopropylethylamine; preferably, the transparent The molar ratio of uric acid to 3-(4-benzylamino)-1,2,4,5-tetrazine is 6-10:1; preferably, the molar ratio of the condensing agent to hyaluronic acid is 0.1- 0.5:1; Preferably, the first solvent includes dimethyl sulfoxide.
[0073] Further, the preparation method further includes: after the amidation reaction, post-treating the reaction product, the molecular weight cut-off of the dialysis bag used for dialysis in the pos...
Embodiment 1
[0090] Step 1: acidify an aqueous solution of sodium hyaluronate (Mw=36KDa) with a strongly acidic ion exchange resin Dowex 50W×8-100. Subsequently, the pH of the filtrate was adjusted to neutral by tetrabutylammonium hydroxide (TBA-OH), and then freeze-dried to obtain the product HA-TBA. HA-TBA and 1,2,4,5-tetrazineamine were dissolved in anhydrous DMSO, then pyBOP and DIEA were added and reacted overnight.
[0091] Among them, the reaction molar ratio of carboxyl group on HA-TBA, 1,2,4,5-tetrazine, pyBOP and DIEA is 8:1:1:1.
[0092] After the reaction in Step 1, the reaction solution was placed in a dialysis bag with a molecular weight cutoff of 3500 Da, dialyzed in deionized water for 1 day, and then purified by G-15 Sephadex chromatography column. Finally, freeze-dry to obtain tetrazine-modified hyaluronic acid (HA-Tz). The structural formula of hyaluronic acid modified by tetrazine is shown in formula (1):
[0093]
[0094] Wherein, the value of n is 115.
[0095]...
Embodiment 2
[0109] Step 1: acidify an aqueous solution of sodium hyaluronate (Mw=36KDa) with a strongly acidic ion exchange resin Dowex 50W×8-100. Subsequently, the pH of the filtrate was adjusted to neutral by tetrabutylammonium hydroxide (TBA-OH), and then freeze-dried to obtain the product HA-TBA. HA-TBA and 1,2,4,5-tetrazineamine were dissolved in anhydrous DMSO, then pyBOP and DIEA were added and reacted overnight.
[0110] Among them, the reaction molar ratio of carboxyl group on HA-TBA, 1,2,4,5-tetrazine, pyBOP and DIEA is 6:1:1:1.
[0111] After the reaction in Step 1, the reaction solution was placed in a dialysis bag with a molecular weight cut-off of 3500 Da, dialyzed in deionized water for 2 days, and then purified by G-15 Sephadex chromatographic column. Finally, freeze-dry to obtain tetrazine-modified hyaluronic acid (HA-Tz). The structural formula of hyaluronic acid modified by tetrazine is shown in formula (1):
[0112]
[0113] Wherein, the value of n is 115.
[01...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com