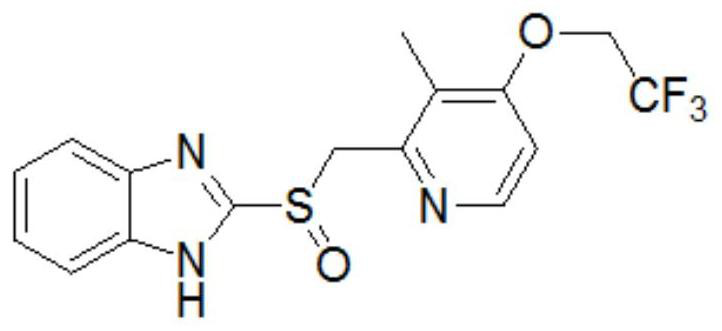
Lansoprazole enteric-coated tablet and preparation method thereof
A technology for enteric and tablet-dissolving lansoprazole, applied in the field of medicine, can solve problems such as affecting the stability of lansoprazole, and achieve the effects of good market prospect, high dissolution rate, and improved bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] 1. Preparation of drug-containing tablet core:
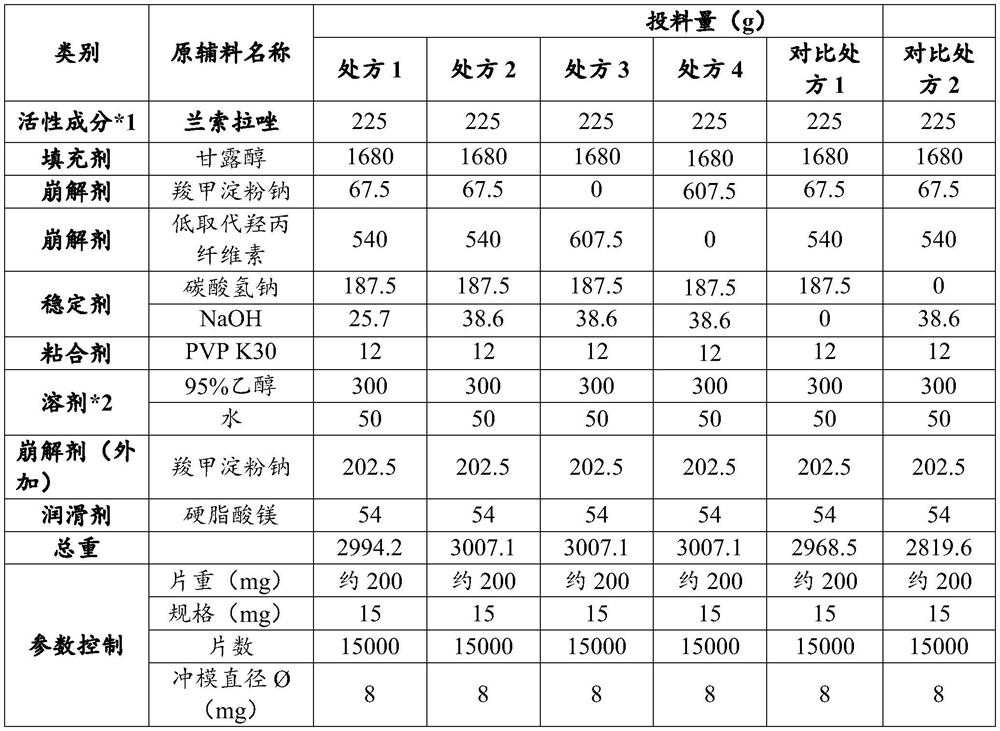
[0062] The prescription composition and process parameters of lansoprazole enteric-coated tablet core are as shown in table 1, and preparation method is as follows:
[0063] Weigh the binder povidone K30 and dissolve it in an appropriate amount of 95% ethanol, stir to dissolve completely, and obtain the binder solution for later use; weigh sodium hydroxide separately, dissolve them in 50ml purified water, and add 200ml 95% ethanol, stir evenly, then add 225g lansoprazole, until dissolving completely (if necessary, can be heated to 25 degrees), for subsequent use; Take by weighing mannitol, sodium bicarbonate, sodium starch glycolate, low Replace hydroxypropyl cellulose, pass through a 60-mesh sieve, and mix with the alkaline solution of lansoprazole in a one-step granulation mixer at a speed of 3r / min for 10min. Then, within 90 seconds, the above-mentioned binder solution was evenly added to the above-mentioned material,...
Embodiment 2
[0083] The dissolution rate of the lansoprazole enteric-coated tablet prepared by the present invention in pH6.8 phosphate buffer saline
[0084] Get the lansoprazole enteric-coated tablet (15mg) that embodiment 1 prepares as test group object, measure according to dissolution rate and release rate assay method (Chinese Pharmacopoeia two general rules 0931 first method method 2), with hydrochloric acid solution (9→ 1000ml) 1000ml is the dissolution medium, the speed is 100 revolutions per minute, operate according to the law, after 120 minutes, immediately lift the basket out of the liquid surface, discard the hydrochloric acid solution, and immediately add phosphoric acid with a pH of 6.0 preheated to 37°C 1000ml of salt buffer solution or phosphate buffer solution of pH 6.8, keep the rotation speed constant, and continue to operate according to the law. After 5, 10, 15, 20, 30, 45 and 60 minutes, take the solution and filter it, and accurately measure the subsequent filtrate ...
Embodiment 3
[0089] Embodiment 3 Lansoprazole enteric-coated tablet is in the stability in accelerated test (40 ± 2 ℃, RH75% ± 5%) and long-term test (25 ± 2 ℃, RH60% ± 10%)
[0090] Make the samples of prescriptions 1-4 and comparative prescriptions 1-2 into market packaging (double aluminum, sealed, carton packaging), and set the test conditions as an accelerated test of 40°C±2°C / 75%RH±5%RH, The investigation time is 6 months, and the detection includes at least 3 time points (such as 0, 3, and 6 months) of the initial and final time, and all investigation items should be included. The results are shown in Tables 5-10.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com