Carbon monoxide conversion process
A carbon monoxide, process technology, applied in the field of carbon monoxide conversion process, to achieve the effect of broadening the use conditions and broadening the source range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The raw material gas is a self-combined gas, in which the volume fraction of hydrogen is 64%, the volume fraction of carbon monoxide is 20%, the volume fraction of carbon dioxide is 10%, and the volume fraction of methane is 12%.
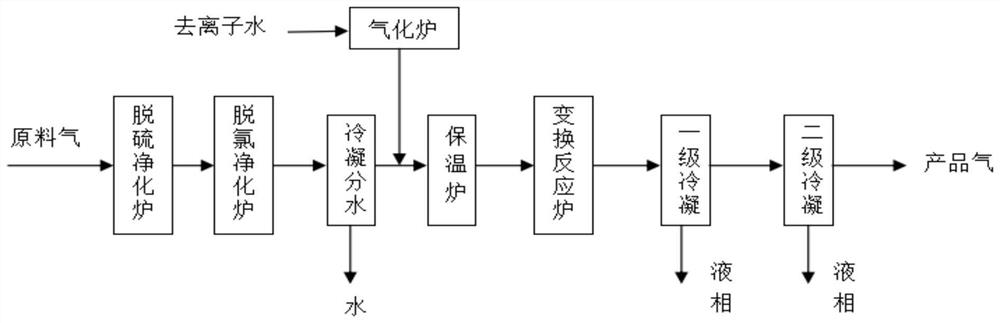
[0039] A schematic diagram of the carbon monoxide shift process is shown in figure 1 , the carbon monoxide shift process comprises the following steps:
[0040] (1) Raw material gas purification: The raw material gas passes through the desulfurization and dechlorination reactors controlled at a pressure of 3.0Mpa and a temperature of 240°C in sequence. The crude desulfurization catalyst A is zinc oxide, and the fine desulfurization catalyst B is a mixture of cobalt-molybdenum catalyst and iron oxide , the volume ratio of A:B is 1.5:1, and after cooling by the water cooling system and gas-liquid separation, the purified feed gas with a sulfide concentration of 0.08ppm and a chloride concentration of 0.04ppm is obtained;
[0041] (2) Water vap...
Embodiment 2
[0047] The raw material gas is coke oven gas, in which the volume fraction of hydrogen is 60%, the volume fraction of carbon monoxide is 10%, the volume fraction of carbon dioxide is 3%, the volume fraction of methane is 18%, and the hydrocarbon is 3%.
[0048] The carbon monoxide shift process includes the following steps:
[0049] 1) Purification of raw material gas: The raw material gas passes through the desulfurization and dechlorination reactors with a pressure of 2.5Mpa and a temperature of 200°C. The crude desulfurization catalyst A is a mixture of zinc oxide and activated carbon, and the fine desulfurization catalyst B is a cobalt-molybdenum catalyst. : B volume ratio is 2.0:1, and then after cooling by water cooling system and gas-liquid separation, the purified raw material gas with sulfide concentration of 0.05ppm and chloride concentration of 0.01ppm is obtained;
[0050] (2) Water vaporization: deionized water is pumped into the vaporizer with a temperature contr...
Embodiment 3
[0056] The raw material gas is the product gas of Inner Mongolia lignite gasification in the laboratory, which contains hydrogen volume fraction of 56%, carbon monoxide volume fraction of 15%, carbon dioxide volume fraction of 14%, methane volume fraction of 5%, and hydrocarbons of 6%.
[0057] The carbon monoxide shift process includes the following steps:
[0058] 1) Purification of raw material gas: The raw material gas passes through the desulfurization and dechlorination reactors with the pressure controlled at 3.0Mpa and the temperature controlled at 240°C. The crude desulfurization catalyst A is a mixture of zinc oxide and activated carbon, and the fine desulfurization catalyst B is a cobalt-molybdenum catalyst and oxidation Iron mixture, the volume ratio of A:B is 1.8:1, after cooling by water cooling system and gas-liquid separation, the purified raw material gas with sulfide concentration of 0.02ppm and chloride concentration of 0.01ppm is obtained;
[0059] (2) Wate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com