Anti-tumor targeted drug sustained-release carrier, preparation and preparation method thereof
A slow-release carrier and slow-release preparation technology, which can be used in anti-tumor drugs, drug combinations, drug delivery, etc., and can solve problems such as intolerance, patients with toxic side effects, and lack of targeting.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1 The preparation method of anti-tumor targeting drug sustained-release carrier
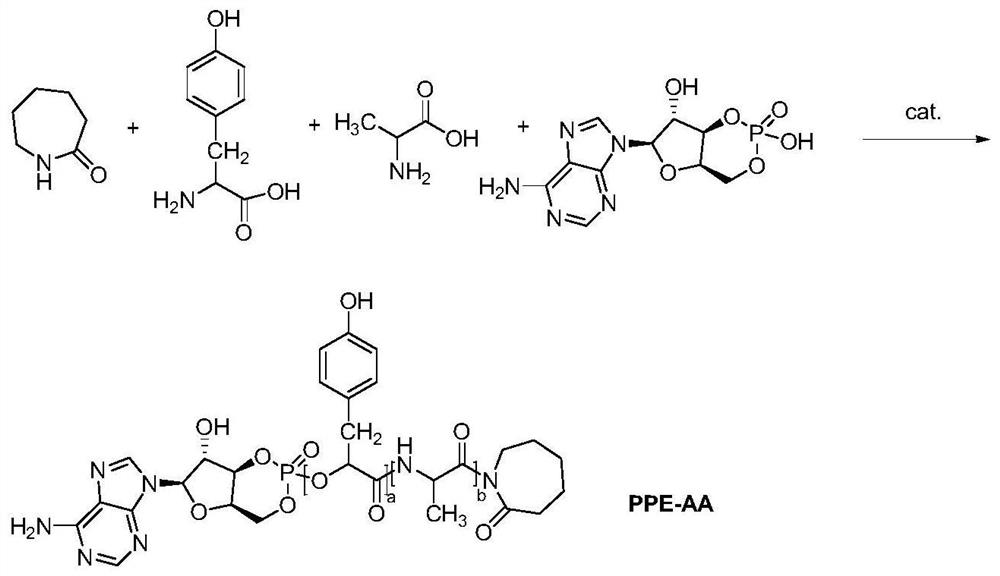
[0036] S1. Preparation of polyphosphate-amino acid copolymer: 1g of alanine, 0.5g of tyrosine, 0.1g of caprolactam, 20mL of deionized water and 0.01g of 1-(3-dimethylaminopropyl)-3-ethyl Add carbodiimide into the reactor, raise the temperature to 180°C under the protection of nitrogen, the reaction time is 0.5h, continue to heat up to 200°C, the reaction time is 1h, then add 0.5g adenosine cyclic phosphate to react for 0.5h, stop Reaction, to be cooled to room temperature to obtain polyphosphate-amino acid copolymer; figure 1 shown;
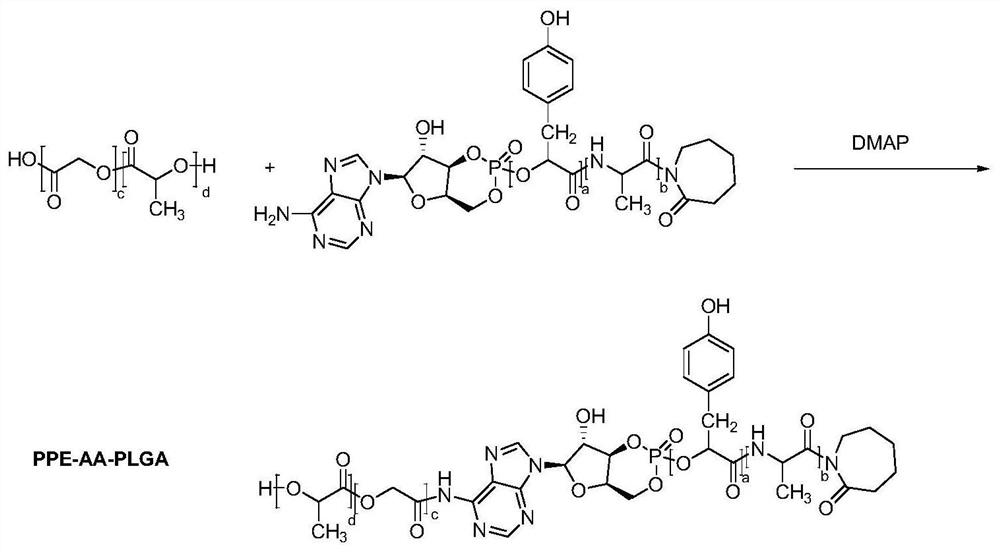
[0037] S2. Configuration of polylactic acid-glycolic acid copolymer solution: weigh 1g of polylactic acid-glycolic acid copolymer, dissolve it in 10mL carbon tetrachloride, and use it as the oil phase;
[0038] S3. Preparation of amphiphile compound: Dissolve 1g of polyphosphate-amino acid copolymer in 10mL of dichloromethane, react for 2h under ...
Embodiment 2
[0041] Example 2 The preparation method of anti-tumor targeted drug sustained-release carrier
[0042] S1. Preparation of polyphosphate-amino acid copolymer (PPE-AA): 1g alanine, 1.2g tyrosine, 0.5g caprolactam, 20mL deionized water and 0.05g dicyclohexylcarbodiimide were added to the reaction In the container, under the protection of nitrogen, the temperature was raised to 200°C, the reaction time was 2h, the temperature was continued to 220°C, the reaction time was 4h, then 0.7g adenosine cyclic phosphate was added to react for 1h, the reaction was stopped, and the polyphosphate was obtained after cooling to room temperature - amino acid copolymers;
[0043] S2. Configuration of polylactic-co-glycolic acid (PLGA) solution: take 1g of poly-lactic-co-glycolic acid, dissolve it in 10mL of dichloromethane, and use it as the oil phase;
[0044] S3. Preparation of amphiphile compound (PPE-AA-PLGA): 1g of polyphosphate-amino acid copolymer was dissolved in 10mL of dichloromethane,...
Embodiment 3
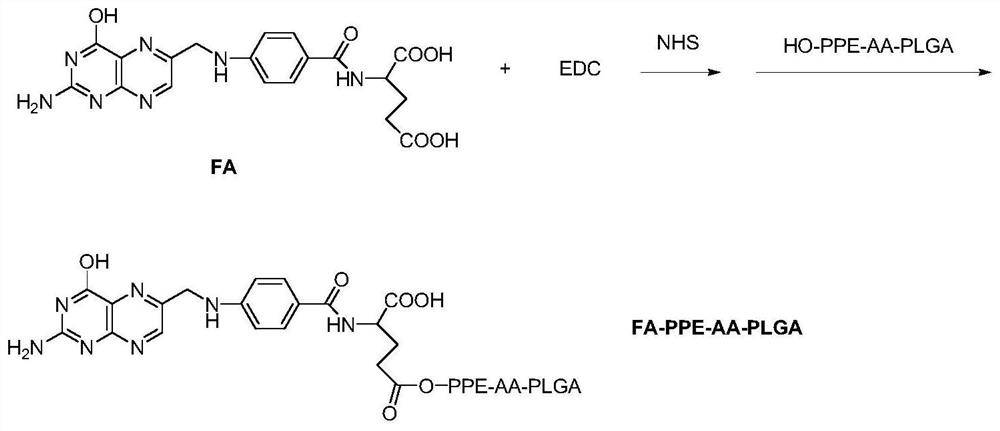
[0048] The difference between this example and Example 2 is that the volume of deionized water added in step S5 in Example 1 is 50 times that of ethyl acetate.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com