Method for breaking C-S bond of alpha-sulfo-arylethanone compound and ligand
A technology for thioaryl ethyl ketone and compound is applied in the field of cleaving C-S bonds, which can solve the problems of high cost and limited substrate, and achieve the effects of simple operation, wide tolerance and low reaction dosage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
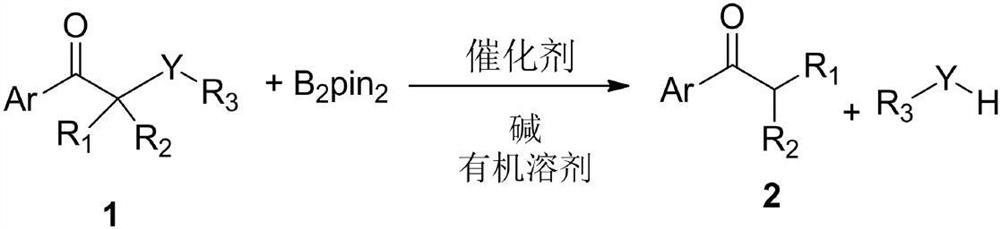
[0045] A method for breaking the C-S bond of α-thioarylethanone compound, the synthesis route of the method is:
[0046]
[0047] The specific operation steps are as follows:
[0048] Add CoCl to a dry reaction tube at 25 °C 2 (0.05mmol), ADI Me (0.05mmol), α-phenylthioacetophenone (1a) (1mmol), biboronic acid pinacol ester (B 2 pin 2 , 1mmol), tetrahydrofuran (THF, 1mL) and methanol (2mmol), finally added sodium tert-butoxide (NaOtBu, 0.15mmol), then stirred at 65 ° C for 1h, separated by column chromatography to obtain acetophenone (1b) , colorless oil, yield 97%.
[0049] The NMR characterization data of acetophenone are:
[0050] 1 H NMR (CDCl 3 ,400MHz): δ8.00-7.94(m,2H),7.60-7.54(m,1H),7.51-7.44(m,2H),2.62(s,3H).
[0051] The NMR characterization data of thiophenol are:
[0052] 1 H NMR (CDCl 3 ,400MHz): δ6.91-7.40(m,5H),3.42(br,1H).
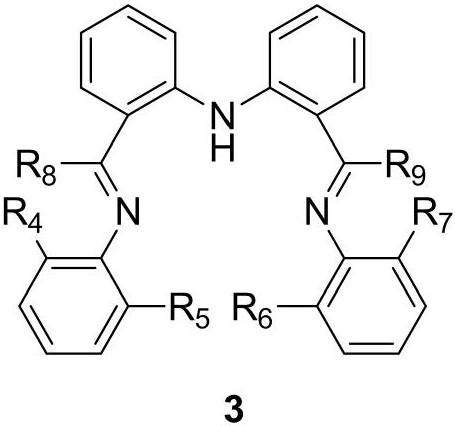
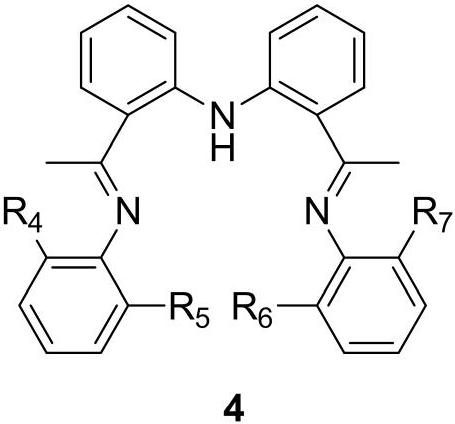
[0053] Among them, ligand ADI Me The preparation method is as follows:
[0054]
[0055] At room temperature, under n...
Embodiment 2-3
[0062] A method for breaking the C-S bond of α-thioarylethanone compound, which is basically the same as that of Example 1, the difference is that ADI is used respectively Et , ADI iPr Replace ADI Me , the yields of acetophenone were 81%, 53%, respectively.
[0063]
[0064]
[0065] ADI Et and ADI iPr ADI Me The preparation process is basically the same, ADI Et and ADI iPr They are also yellow solids, with melting points of 68-69°C and 73-74°C, and yields of 80% and 71%, respectively.
[0066] ADI Et The NMR characterization data of is:
[0067] 1 H NMR (CDCl 3 ,400MHz):δ12.12(br,1H),7.73(d,J=8.0Hz,2H),7.44-7.35(m,2H),7.33-7.25(m,2H),7.00-6.93(m,2H ),6.90-6.82(m,4H),6.79-6.73(m,2H),2.82(t,J=7.2Hz,8H),2.05(s,6H),1.22(d,J=7.2Hz,12H) .
[0068] ADI iPr The NMR characterization data of is:
[0069] 1 H NMR (CDCl 3 ,400MHz):δ12.01(br,1H),7.69(d,J=8.0Hz,2H),7.42-7.33(m,2H),7.30-7.24(m,2H),6.99-6.91(m,2H ),6.88-6.82(m,4H),6.77-6.70(m,2H),2.91-8.82(m,4H),2.02(...
Embodiment 4-5
[0071] A method for breaking the C-S bond of α-thioarylethanone compound, which is basically the same as that of Example 1, the difference is that NiCl is used in Examples 4-5 respectively 2 , FeCl 2 Replace CoCl 2 , the yields of acetophenone were 80%, 59%, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com