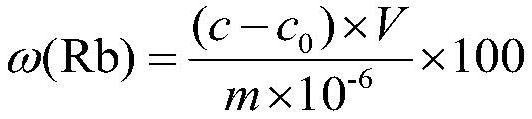Chemical phase analysis method for rubidium in metal ore
A metal ore and phase analysis technology, applied in thermal excitation analysis, material excitation analysis, etc., to achieve the effect of wide linear range, good reproducibility, and small background interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The mensuration of embodiment 1 rubidium content
[0026] The beneficiation of a mining area enriches rubidium-containing mica, rubidium in fluorite, silicon-aluminum oxides and rubidium and refractory minerals in feldspar, and there are many types of polymetallic minerals. For such minerals with a very close symbiotic relationship and complex ore material components, the method of this embodiment can be used to determine the total rubidium content in the sample, the rubidium content in mica, the rubidium content in iron and aluminum oxides, silicates and other minerals. Rubidium content in minerals, rubidium content in fluorite.
[0027] The mensuration of total rubidium content of present embodiment: take by weighing 0.1 gram of sample in the plastic king beaker, add a small amount of water wetting, add mixed acid: 10ml hydrochloric acid (36%~38%), 3~5ml nitric acid (65%~68%) %), 5ml hydrofluoric acid (≥40%) and 5mL perchloric acid (70%~72%), heated to 270-280°C for ...
Embodiment 2
[0042] Embodiment 2 precision and actual sample test
Embodiment 1
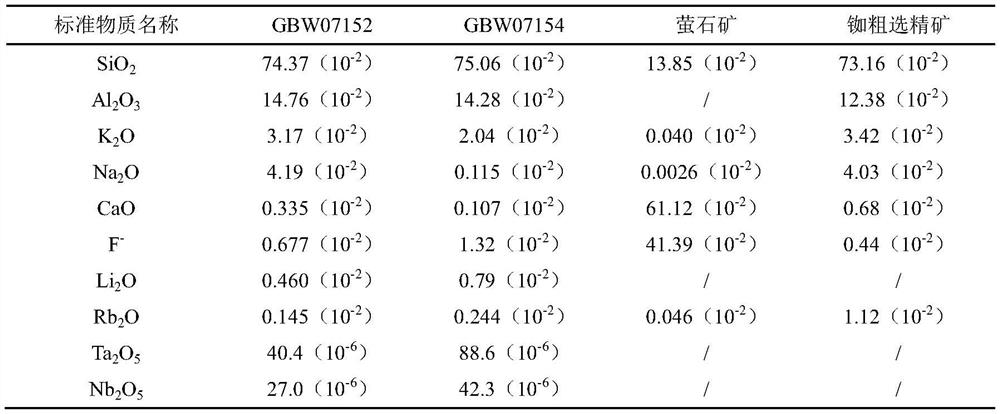
[0043]For the precision of the determination of rubidium content by the ICP-OES method in Example 1, use the national standard materials lithium ore GBW07152, tantalum ore GBW07154 and self-made standard samples of rubidium roughing concentrate and fluorite ore to calculate the results of 11 parallel measurements , the main components of the reference material are shown in Table 1.
[0044] Table 1 Main Components of Standard Substances
[0045]
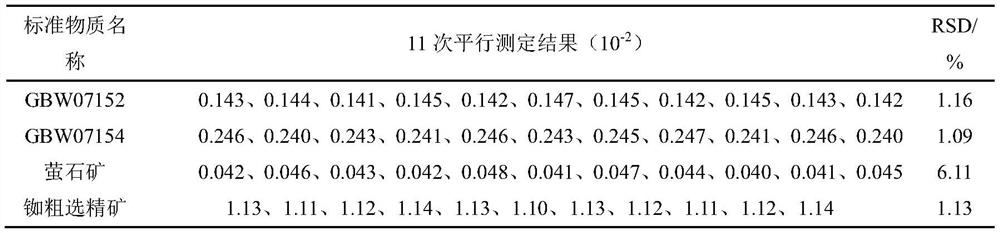
[0046] The results of 11 parallel determinations of four samples are shown in Table 2:
[0047] Table 2 Precision Test
[0048]
[0049] The test results show that the method of the invention has high precision and wide application range.
[0050] Adopt the method of embodiment 1 to test 6 samples in a certain mining area, the result data is as table 3, and the customer feedback is very good:
[0051] table 3
[0052]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com