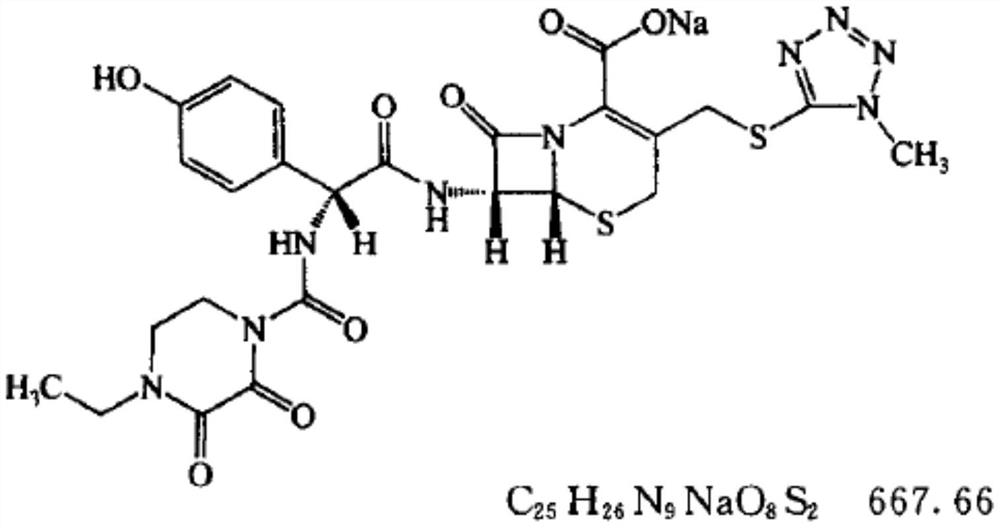
Preparation method of cefoperazone sodium
A technology of cefoperazone sodium and cefoperazone acid, which is applied in the field of medicine, can solve the problems of solvents and residues that cannot be effectively solved, and achieve the effects of low acetone solvent residues, uniform particles, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
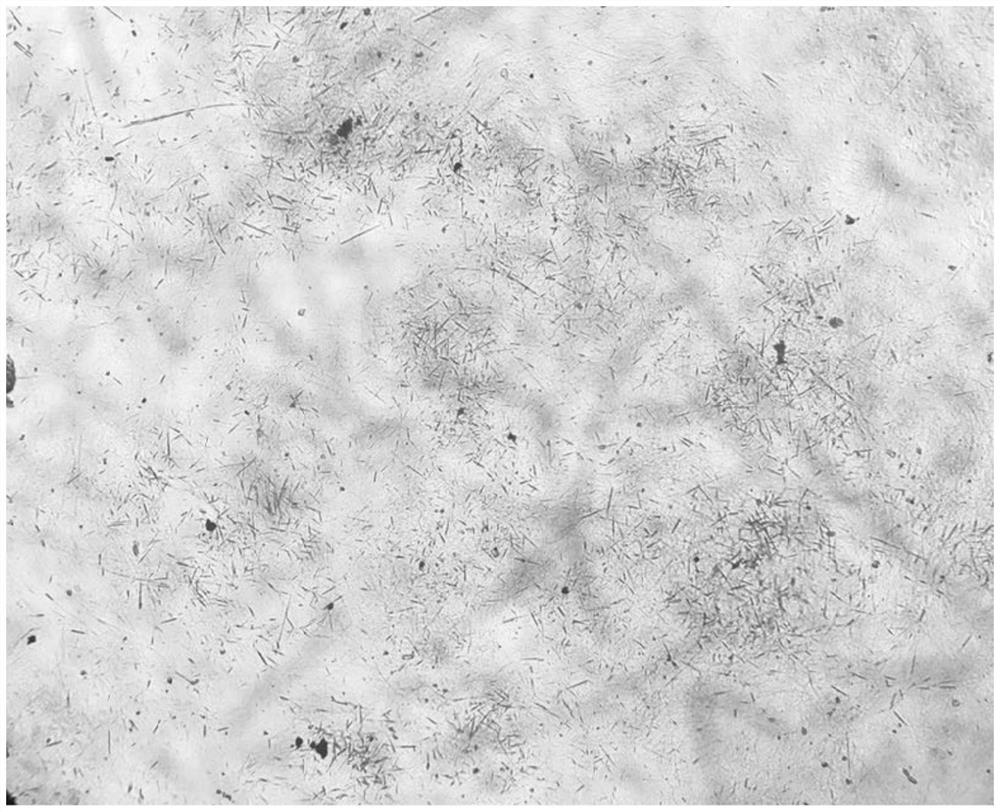
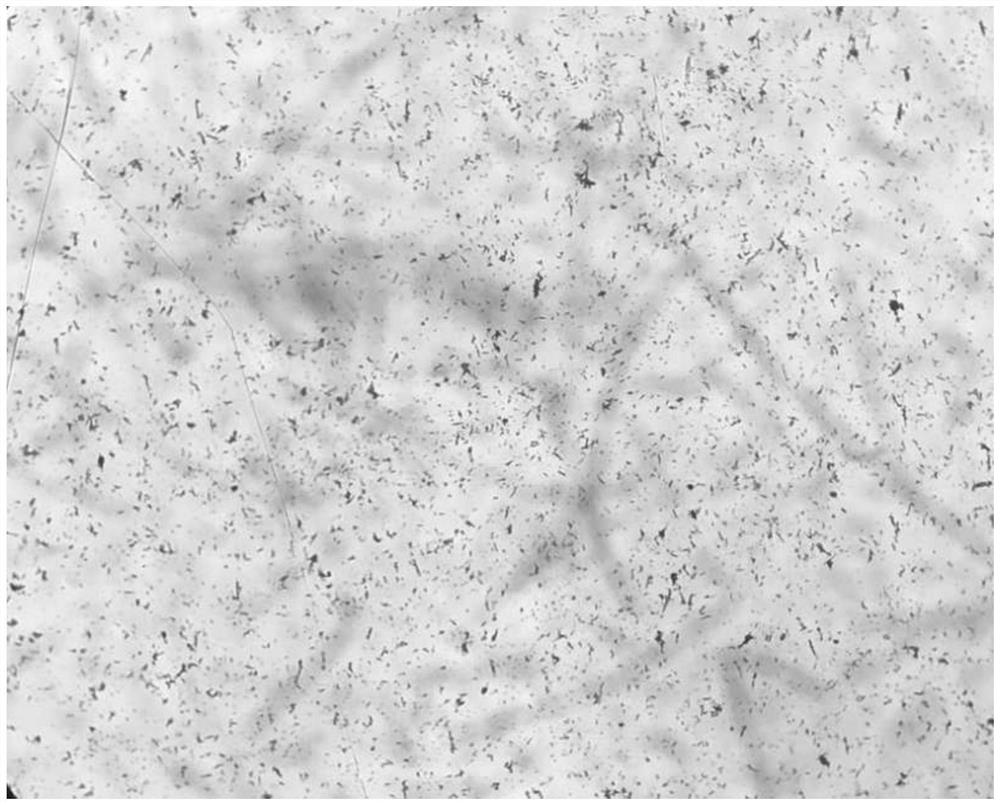
Image
Examples
Embodiment 1
[0033] Add 50ml of acetone to the reaction tank, lower the temperature to 0°C, add 50g of cefoperazone acid, stir and control the temperature at 0°C; add 10ml of purified water to another dissolving tank, control the temperature at 60°C, add 6.5g of sodium bicarbonate, stir to dissolve Clear; add the lye stream to the cefoperazone acid solution, control the temperature at 0°C, and press it into the crystallization tank through the sterilizing filter line after the stream is added;
[0034] Pass sterile nitrogen and acetone into the crystallization tank through the gas-liquid two-phase nozzle, keep the acetone flow rate at 3ml / min, and the nitrogen pressure at 5MPa; when the acetone flow reaches 250ml, add 0.5g of seed crystal and grow the crystal for 15min; Add nitrogen and acetone to the liquid two-phase nozzle to keep the flow rate and pressure constant; after adding acetone to 750ml, cool down and grow the crystal.
[0035] Filtration, vacuum drying, discharging, obtain cef...
Embodiment 2
[0037] Add 75ml of acetone to the reaction tank, lower the temperature by 10°C, add 50g of cefoperazone acid, stir and control the temperature at 10°C; add 13ml of purified water to another dissolution tank, control the temperature at 70°C, add 7.1g of sodium bicarbonate, stir to Clear; add the lye stream to the cefoperazone acid solution, control the temperature at 10°C, and press it into the crystallization tank through the sterilizing filter line after the stream is added;
[0038] Pass sterile nitrogen and acetone into the crystallization tank through the gas-liquid two-phase nozzle, keep the acetone flow rate at 5ml / min, and the nitrogen pressure at 5MPa; Add nitrogen and acetone to the liquid two-phase nozzle to keep the flow rate and pressure constant; after adding acetone to 100ml, cool down and grow the crystal.
[0039] Filtrate, vacuum dry, and discharge to obtain 47.7 g of cefoperazone sodium product.
Embodiment 3
[0041] Add 65ml of acetone to the reaction tank, lower the temperature by 5°C, add 50g of cefoperazone acid, stir and control the temperature at 5°C; add 11ml of purified water to another dissolution tank, control the temperature at 65°C, add 6.8g of sodium bicarbonate, stir to Clear; add the lye stream to the cefoperazone acid solution, control the temperature at 5°C, and then press it into the crystallization tank through the sterilizing filter line after the stream is added;
[0042] Pass sterile nitrogen and acetone into the crystallization tank through the gas-liquid two-phase nozzle, keep the acetone flow rate at 4ml / min, and the nitrogen pressure at 5MPa; Add nitrogen and acetone to the liquid two-phase nozzle to keep the flow rate and pressure constant; after adding acetone to 850ml, cool down and grow the crystal.
[0043] Filtrate, vacuum dry, and discharge to obtain 47.2 g of cefoperazone sodium product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com