Ivabradine hydrochloride sustained-release preparation as well as preparation method and application thereof
A technology for ivabradine hydrochloride and sustained-release preparations, which is applied in the field of ivabradine hydrochloride sustained-release preparations and its preparation, and can solve problems such as greater influence on drug release and absorption, difficulty in industrial production, and unfavorable drug stability , to avoid the effect of drug burst release, reduce the incidence of adverse reactions, and reduce the number of times of medication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] (1) Drug-containing layer (per 2000 capsules)
[0053]
[0054] (2) Isolation coat layer (per 2000 capsules)
[0055] Opadry YS-1-7003 3.0g
[0056] Purified water 27g
[0057] (3) Sustained-release coating (per 2000 capsules)
[0058] Surelease water dispersion 70.4g
[0059] PEG4000 2.2g
[0060] Purified water 47g
[0061] (4) Protective coat layer (per 2000 capsules)
[0062] Opadry YS-1-7003 5.2g
[0063] Purified water 46.8g
[0064] Embodiment 1——Example 7 ivabradine hydrochloride sustained-release preparation preparation process:
[0065] (1) Add the prescribed amount of ivabradine hydrochloride crude drug and surfactant into pure water in a certain proportion, and fully stir until the solid drug is completely dissolved;
[0066] (2) Add Opadry YS-1-7003 to the above-mentioned drug-containing solution, and stir well to obtain a drug-containing suspension;
[0067] (3) Put a certain amount of blank pellet cores in the bottom spraying device of the fl...
Embodiment 2
[0075] Drug-containing layer (per 2000 capsules)
[0076]
[0077] Other auxiliary materials category and dosage and preparation process are all the same as in Example 1.
Embodiment 3
[0079] Drug-containing layer (per 2000 capsules)
[0080]
[0081] Other auxiliary materials category and dosage and preparation process are all the same as in Example 1.
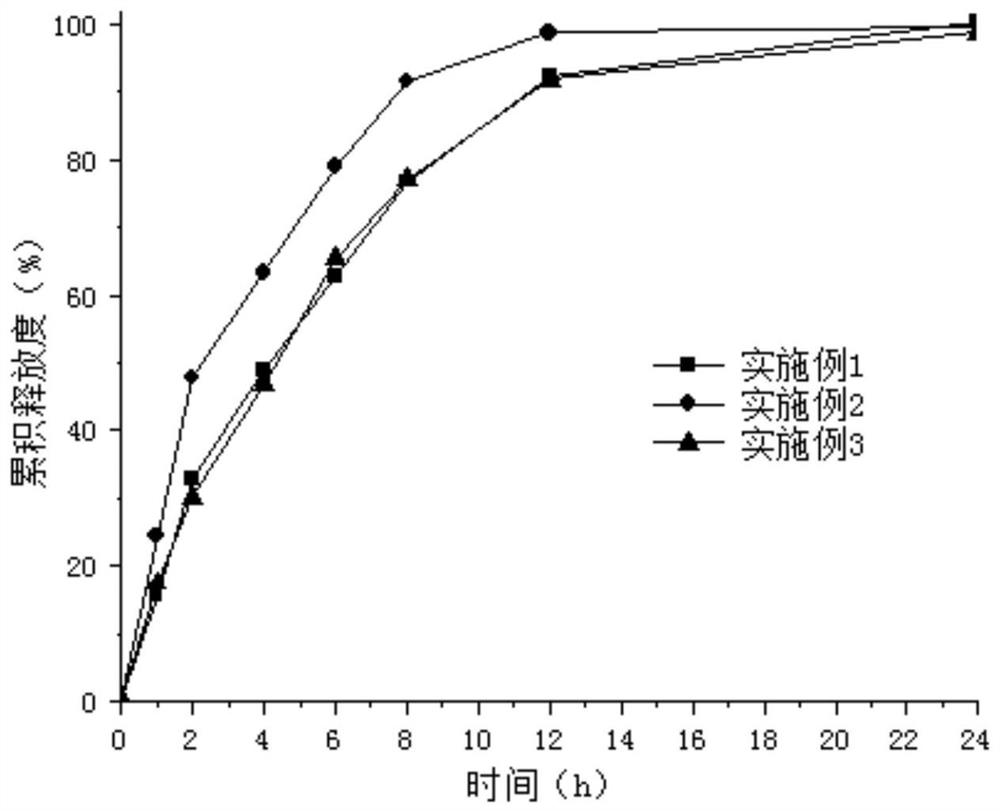
[0082] For comparison of the release curves of ivabradine hydrochloride sustained-release preparations in Examples 1 to 3, see figure 1 . The results showed that the release curves of the sustained-release capsules prepared with sucrose cores showed a burst release phenomenon, while the release curves of the sustained-release capsules prepared with microcrystalline cellulose cores and starch cores were normal. Since the sucrose core is easily soluble in water, it is easy to generate osmotic pressure under the condition of aqueous coating to make the drug migrate to the sustained-release layer, resulting in burst release. Compared with the starch core, the hardness of the microcrystalline cellulose pellet core is larger, the friability is lower, and the pellet core is less likely to be broken during the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com