Yellow fluorescent carbon dot as well as preparation method and application thereof
A yellow fluorescence, fluorescent carbon dot technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of weak emission, limited application of carbon dots, biological tissue damage, etc., and achieve stable optical properties and good detection. effect, good solubility and dispersibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Step 1, at room temperature, dissolve 190.1 mg of EGTA and 108.1 mg of p-phenylenediamine in 10 mL of deionized water, stir well, and sonicate to obtain a clear solution.
[0033] Step 2, transfer the solution to a 25mL hydrothermal reaction kettle.
[0034] Step 3: Put the hydrothermal kettle in an oven and react at 200° C. for 5 hours to obtain a brown solution.
[0035] Step 4, take out the hydrothermal reaction kettle, cool naturally, and centrifuge the obtained carbon dot solution at 10000rpm for 10min to remove insoluble matter. Further filter with a 0.22 μm microporous membrane.
[0036] In step 5, the above-mentioned fluorescent carbon quantum dot aqueous solution is freeze-dried to obtain yellow fluorescent carbon dots.
Embodiment 2
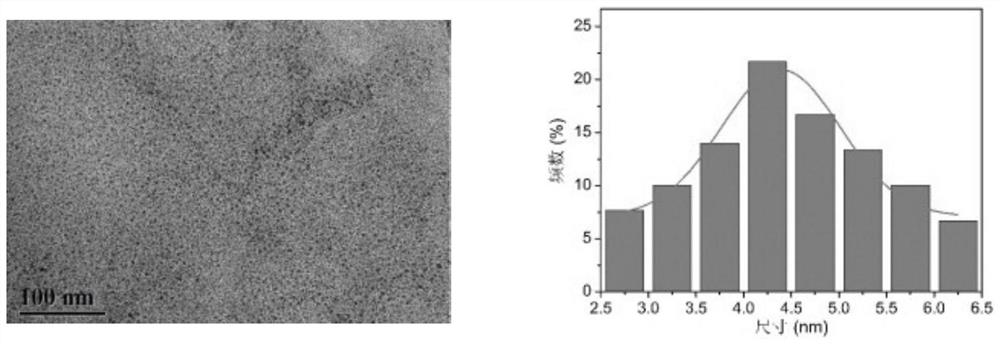
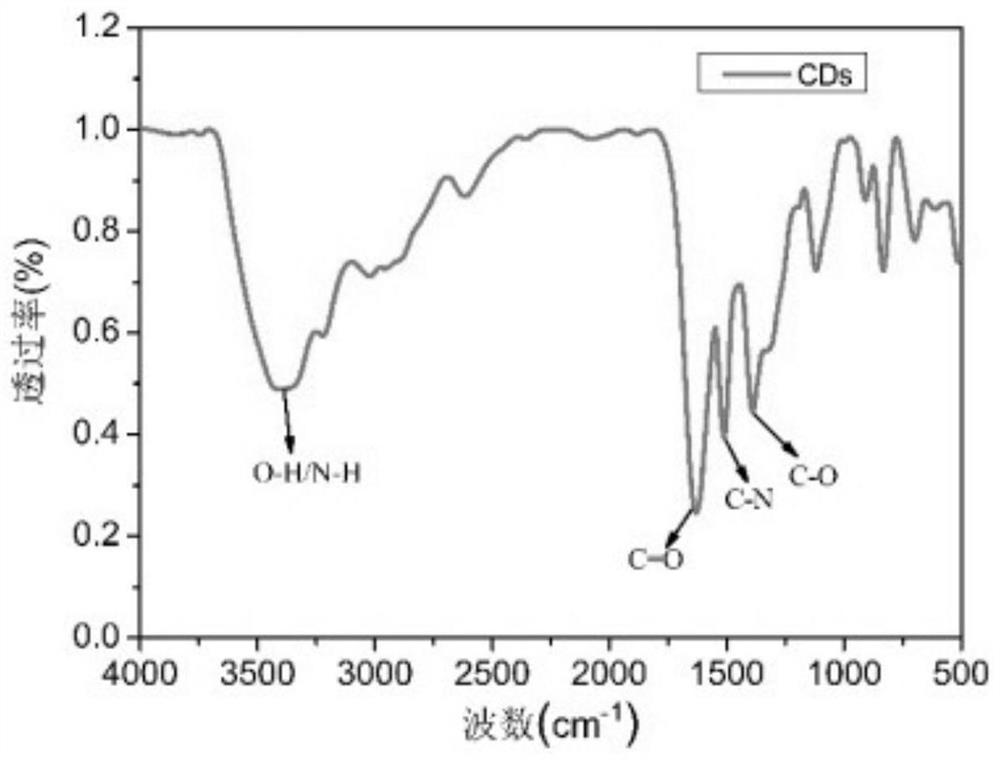
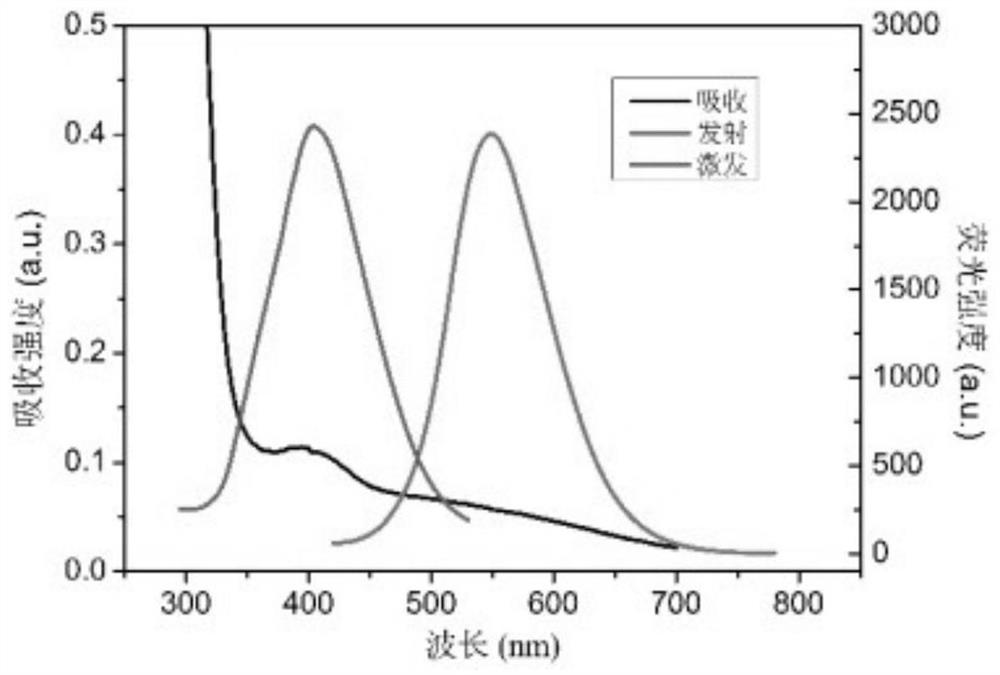
[0038] The carbon dot prepared in embodiment 1 is carried out TEM and infrared spectroscopic characterization (see Figure 1-2 ), for UV absorption spectroscopy and fluorescence excitation emission characterization (see image 3 ), the particle size of carbon dots prepared by the present invention is all less than 10nm, and the surface contains groups such as carboxyl, hydroxyl, amino groups, and emits yellow fluorescence.
Embodiment 3
[0040] Get 1mL of the carbon dot aqueous solution (0.30mg / mL) prepared in Example 1 and place it in a fluorescent cuvette, add 10 μL concentration of chlortetracycline solution of 1, 5, 10, 15 ... 95, 100 μM respectively, mix well, Scan the emission spectrum (λex=400nm, λem=540nm) in the fluorescence photometer, according to the relationship between the concentration of aureomycin and the relative fluorescence intensity change value, calculate the detection range and detection limit of carbon dots to aureomycin ( See Figure 4 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com