Oxytetracycline hydrochloride microbial limit inspection method
An oxytetracycline hydrochloride, microbial limit technology, applied in the directions of microorganism-based methods, microbial determination/inspection, biochemical equipment and methods, etc., can solve problems such as difficulty in carrying out microbial limit testing, short dissolution time, and impact on test results. , to achieve the effect of improving colony recovery rate, good dissolution effect and high detection accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: adopt prior art to prepare sample solution:
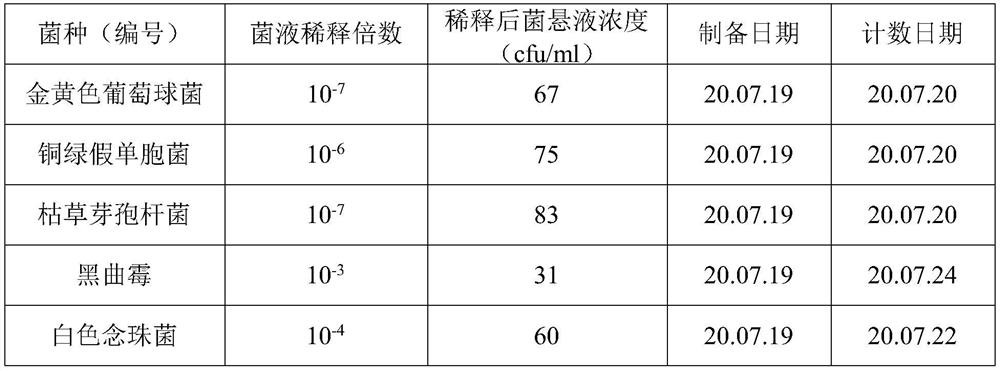
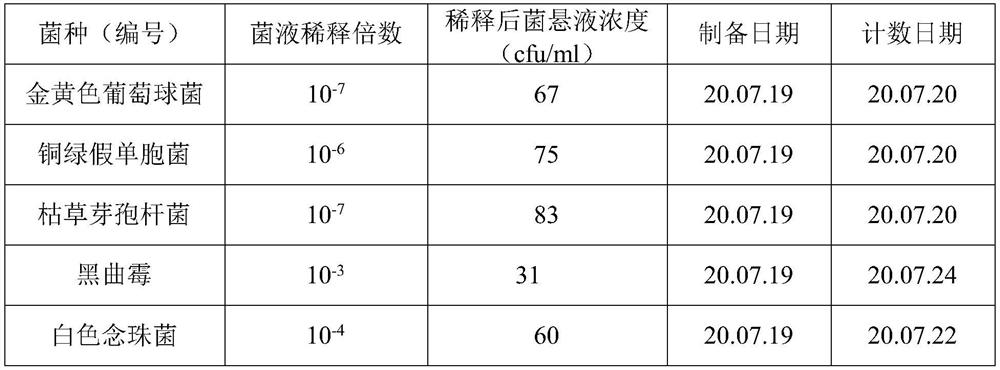
[0031] On the ultra-clean workbench in the clean room, add 10g of oxytetracycline hydrochloride sample into a 250ml stoppered Erlenmeyer flask, accurately measure 100ml of sterilized water with a sterilized measuring cylinder, shake fully, and use it as a 1:10 sample solution;
[0032] From the 1:10 sample solution, use a sterilized tip to accurately draw 10ml into a 250ml conical flask, then use a sterilized graduated cylinder to measure 90ml of sterilized water into the conical flask, shake it fully, and use it as 1: 100 of the sample solution.
[0033] From the 1:100 sample solution, use a sterilized tip to accurately draw 10ml into a 250ml conical flask, then use a sterilized graduated cylinder to measure 90ml of sterilized water into the conical flask, shake it fully, and use it as 1: 1000 of the sample solution.
[0034] From the 1:1000 sample solution, use a sterilized tip to accurately draw 10ml into ...
Embodiment 2
[0035] Embodiment 2: diluent is selected the sample solution preparation of 20% sterile polyethylene glycol 200 for use
[0036] On the ultra-clean workbench in the clean room, add 10g of oxytetracycline hydrochloride sample into a 250ml Erlenmeyer flask with a stopper, accurately measure 200 to 100ml of 20% sterile polyethylene glycol with a sterilized measuring cylinder, shake fully, and use as 1 : 10 sample solutions;
[0037] From the 1:10 sample solution, use a sterilized tip to accurately draw 10ml into a 250ml conical flask, then use a sterilized graduated cylinder to measure 90ml of 20% sterile polyethylene glycol 200 into the conical flask, Shake well, as a 1:100 sample solution.
[0038] From the 1:100 sample solution, use a sterilized tip to accurately draw 10ml into a 250ml conical flask, then use a sterilized graduated cylinder to measure 90ml of 20% sterile polyethylene glycol 200 into the conical flask, Shake well, as a 1:1000 sample solution.
Embodiment 3
[0039] Embodiment 3: diluent is selected the sample solution preparation of 20% aseptic glycerol formal
[0040] Put 10g of oxytetracycline hydrochloride sample into a 250ml Erlenmeyer flask with a stopper on the ultra-clean workbench in a clean room, accurately measure 20% sterile glycerin formal to 100ml with a sterilized measuring cylinder, and shake it fully, as 1:10 the sample solution;
[0041] From the 1:10 sample solution, use a sterilized tip to accurately draw 10ml into a 250ml conical flask, then use a sterilized measuring cylinder to measure 90ml of 20% sterile glycerol formal into the conical flask, shake fully Shake, as a 1:100 sample solution.
[0042] From the 1:100 sample solution, use a sterilized tip to accurately draw 10ml into a 250ml conical flask, then use a sterilized graduated cylinder to measure 90ml of 20% sterile glycerol formal into the conical flask, shake fully Shake, as a 1:1000 sample solution.
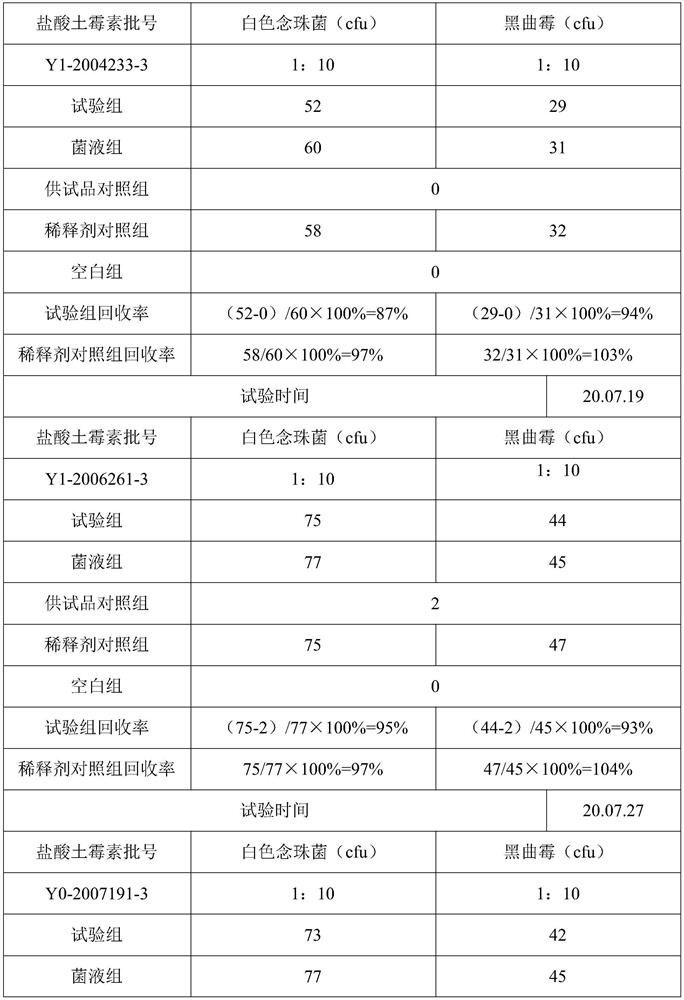
[0043] 2. Verification of mold and yeast test...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap