Probe, primer and kit for detecting gene polymorphism of tacrolimus individualized medication
A technology of gene polymorphism and tacrolimus is applied in the field of probes, primers and kits for detecting gene polymorphism of tacrolimus individualized medicine, which can solve the problem of high cost, inappropriate detection, time-consuming and labor-intensive, etc. problem, to achieve the effect of simple operation, clear genotyping and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: probe and primer design
[0028] The present invention designs probe and primer sequences for the CYP3A5 677A>G SNP site. The specific principle is to use the conformational change of the fluorescent probe and the target sequence after hybridization to release the fluorescent dye, and judge the genotyping results according to the peak diagrams and Tm values at different temperatures after hybridization. In the absence of target DNA, the fluorophore and quencher can be stably combined together, and no fluorescent signal can be detected; when there is target DNA, the structure of the fluorescently labeled probe is destroyed, and the fluorophore and quencher The extinguishing groups are separated from each other, and the fluorescent signal can be detected.
[0029] Design the probe and primers so that when the template does not exist at the annealing temperature, the probe is in a stem-loop state, including the loop sequence and the reverse-complementary s...
Embodiment 2
[0036] Embodiment 2: Detect different genotype standard products
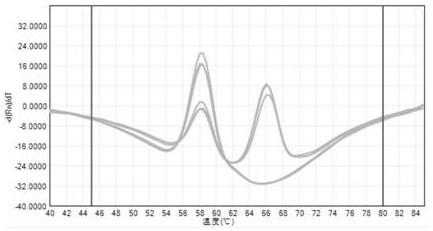
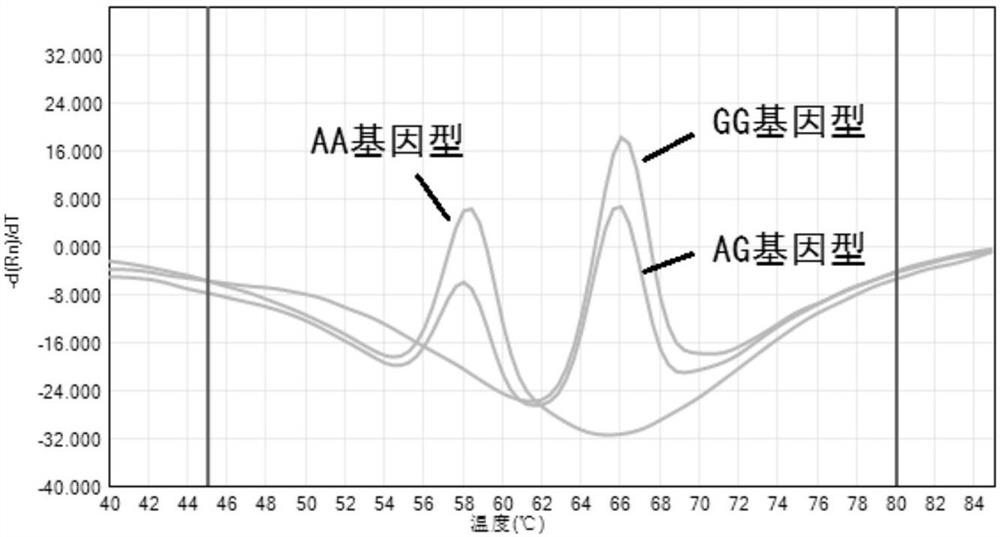
[0037] 1. Use the plasmid CYP3A5 6986 to construct and prepare wild-type standard plasmids and mutant standard plasmids containing the rs776746 site of the target gene (the source of the plasmid, and the synthesis of the plasmid containing the target gene were synthesized by Sangon Bioengineering (Shanghai) Co., Ltd. .The accuracy of the sequence was determined by sanger sequencing. The genotype of the wild-type standard plasmid rs776746 was AA;
[0038] 2. Using the probes and primers in Example 1.
[0039] 3. PCR reaction system:
[0040] 1) Add 7.5ul of PCR Mix, 0.5uM of primer 1 solution and 0.5uM of primer 2 solution, and 0.1uM of probe into each PCR reaction well, and then add the wild-type standard plasmid into 3 different PCR reaction wells respectively 2ul each of DNA, mutant standard plasmid DNA, mixed DNA (wild type standard plasmid and mutant standard plasmid are mixed at a ratio of 1:1), make up ...
Embodiment 3
[0043] Embodiment 3: detection method performance analysis experiment
[0044] 1. Precision experiment
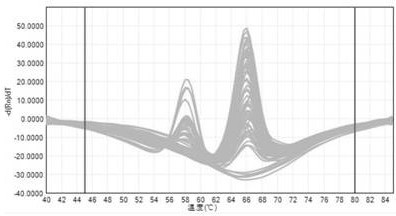
[0045] Take a copy of wild-type standard plasmid DNA and mixed-type DNA (the wild-type standard plasmid and mutant standard plasmid are mixed at a ratio of 1:1), and test 3 times a day for 5 consecutive days. The method in example 2, see the result figure 2 . It shows that the fluorescent PCR amplification reaction of the present invention has good repeatability (the coincidence rate is greater than 95%, and the variation coefficient CV of the AG value of the detection result is less than 5%).
[0046] 2. Conformity rate experiment
[0047] Select 20 cases of DNA samples from healthy volunteers in Shanghai, apply the method in Example 2 to detect the rs776746 site, and use the Sanger sequencing method to verify at the same time, compare the consistency of the detection results of the two methods, and the results show that the method in Example 2 can be classified The c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com