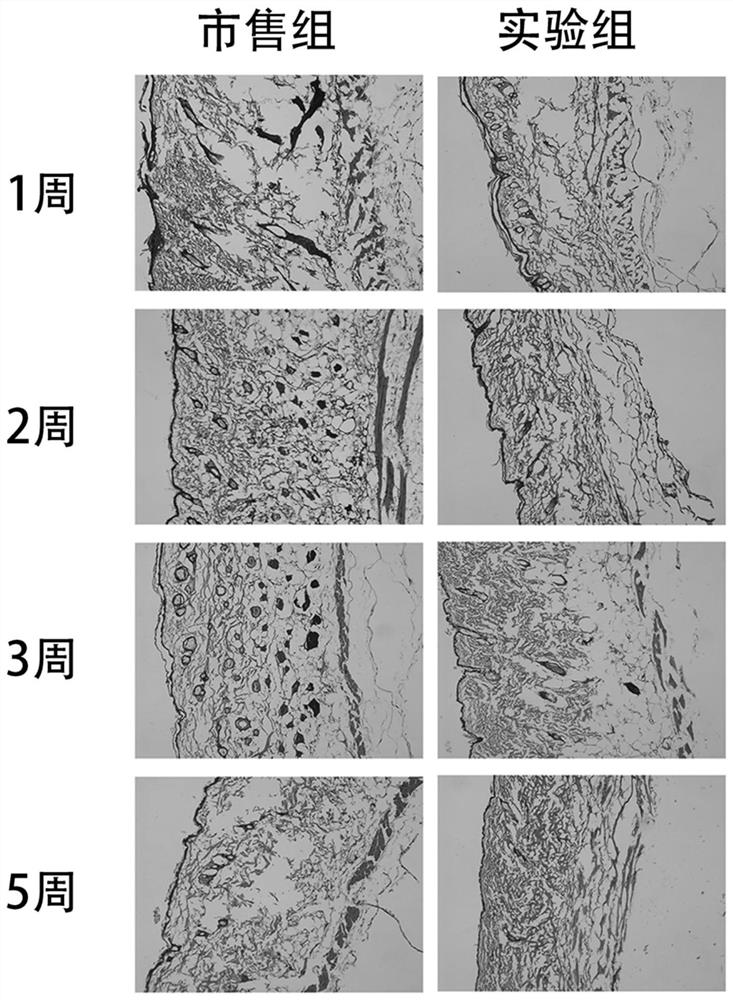
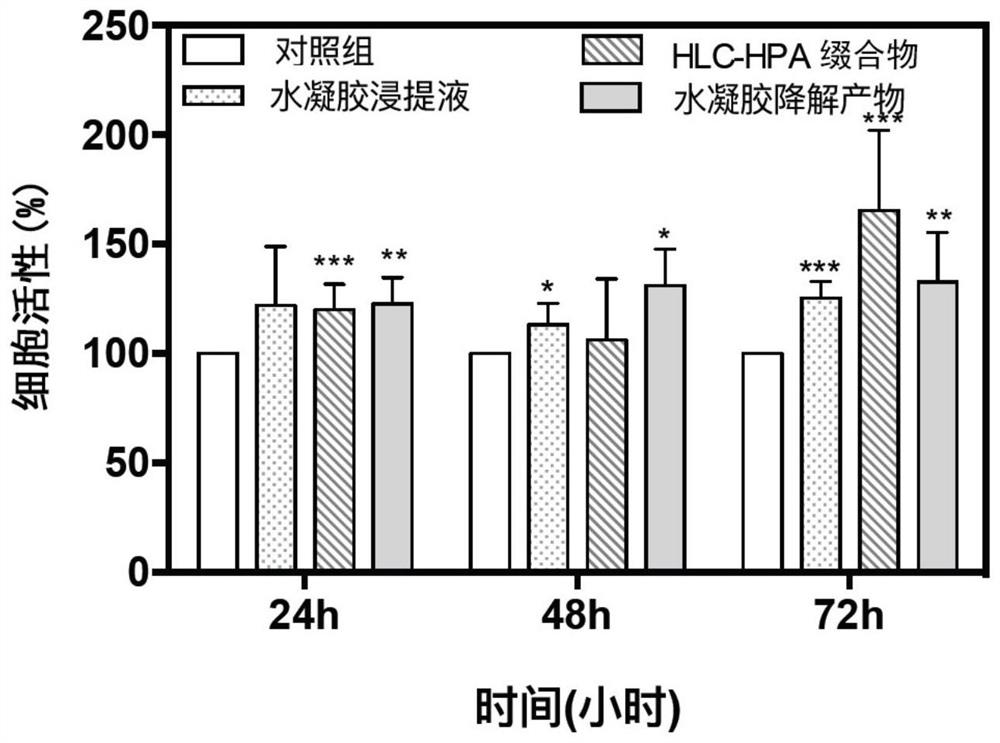
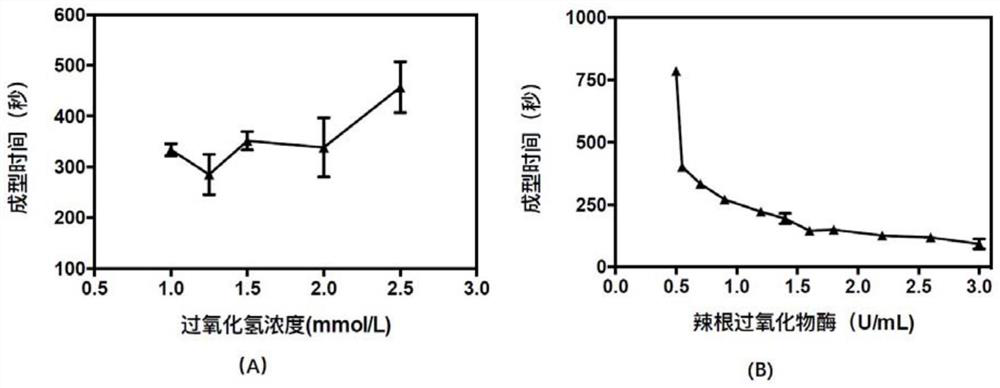
Recombinant collagen injectable hydrogel and preparation method thereof
A technology for recombining collagen and water for injection, which is applied in pharmaceutical formulations, prostheses, aerosol delivery, etc., can solve problems such as human injury and achieve high cross-linking rate, excellent biocompatibility and biodegradability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] The application provides a method for preparing recombinant collagen injectable hydrogel, which is characterized in that it comprises the following steps:
[0036] Respectively adding a certain concentration of p-hydroxyphenylpropionic acid, an activator and a recombinant collagen solution into the solvent to form a mixed solution, under the action of the activator, the recombinant collagen and p-hydroxyphenylpropionic acid undergo a dehydration condensation reaction;
[0037] Separating the mixed solution after the above reaction - removing unreacted components and then lyophilizing to obtain a recombinant collagen-p-hydroxyphenylpropionic acid conjugate;
[0038] Dispersing the above-mentioned recombinant collagen-p-hydroxyphenylpropionate conjugate in an aqueous phase to obtain a precursor solution; and
[0039] adding hydrogen peroxide and peroxidase to catalyze the precursor solution to form a hydrogel in situ;
[0040] Wherein, the recombinant collagen consists o...
Embodiment 1
[0066] Step 1: 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, N-hydroxysuccinimide and p-hydroxyphenylpropionic acid were mixed with 2.5% (w / v) , 3.0% (w / v) and 1.3% (w / v) were dissolved in 250mL of 40% (v / v) aqueous solution of dimethylformamide, stirred at room temperature for 12 hours;
[0067] Step 2: Add 150 mL of 8.5% (w / v) recombinant collagen solution to the above solution, and stir overnight;
[0068] Step 3: Transfer the above reaction solution to a dialysis bag with a cut-off molecular weight of 8000-12000 Da, lyophilize after dialysis to obtain a recombinant collagen-p-hydroxyphenylpropionic acid conjugate;
[0069] Step 4: Dissolve the recombinant collagen-p-hydroxyphenylpropionic acid conjugate in water for injection at a concentration of 50mg / mL, add hydrogen peroxide and horseradish peroxide to make the final concentrations 1.5mmol / L and 1.0mmol / L respectively U / mL, hydrogels formed in situ.
Embodiment 2
[0071] Step 1: 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, N-hydroxysuccinimide and p-hydroxyphenylpropionic acid were added at 5.0% (w / v) , 2.5% (w / v) and 0.8% (w / v) were dissolved in 200mL of 40% (v / v) dimethylformamide aqueous solution, stirred at room temperature for 10 hours;
[0072] Step 2: Add 150 mL of 10.0% (w / v) recombinant collagen solution to the above solution, and stir overnight;
[0073] Step 3: Transfer the above reaction solution to a dialysis bag with a cut-off molecular weight of 8000-12000 Da, lyophilize after dialysis to obtain a recombinant collagen-p-hydroxyphenylpropionic acid conjugate;
[0074] Step 4: Dissolve the recombinant collagen-p-hydroxyphenylpropionic acid conjugate in water for injection at a concentration of 50mg / mL, add hydrogen peroxide and horseradish peroxidase to make the final concentrations 2.0mmol / L and 1.0U / mL, form hydrogel in situ.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com