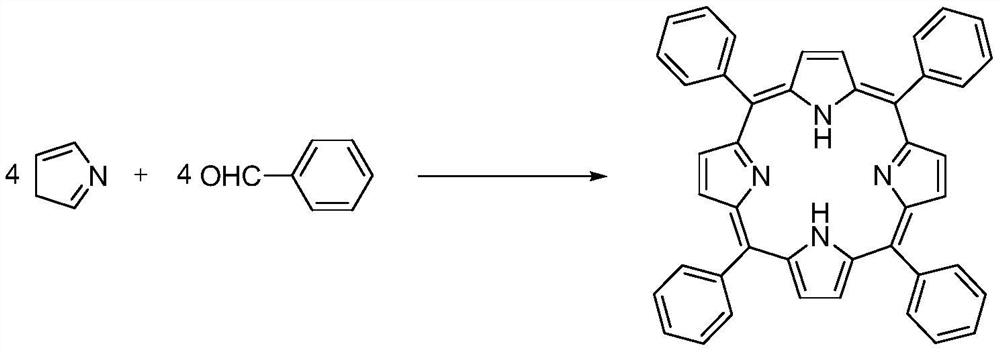
Method for synthesizing tetraphenylporphyrin by using return pipe type reactor
A technology of tubular reactor and tetraphenylporphyrin, applied in the direction of organic chemistry, can solve the problems of increasing the three wastes and regional reaction selectivity, using a large amount of catalyst, sticky acidic filtrate, etc., so as to avoid contact with toxic and harmful substances , Avoid raw material recovery process, avoid the effect of tar
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
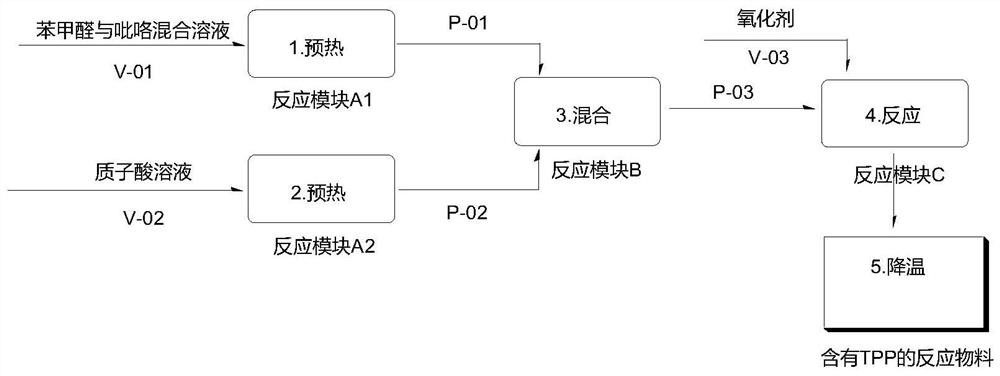
[0033] 1) After the two reaction modules A1 and A2 are connected in parallel, they are connected in series with the two reaction modules B and C in sequence, and the microchannel reactor is replaced with a protonic acid; 106g (1mol) of benzaldehyde and 67g (1mol) of pyrrole are weighed and mixed into a homogeneous solution Put it into storage tank V-01, with a volume of about 170mL; weigh 680g of propionic acid and put it into storage tank V-02, with a volume of about 681mL;
[0034] 2) Control the volume flow rate of the mixed solution of benzaldehyde and pyrrole to 2.0mL / min, enter the first reaction module A1 by pump P-01 to preheat at 110°C; control the volume flow rate of propionic acid to 7.8mL / min by pump P- 02 Enter the second reaction module A2 to preheat 120°C; then enter the third reaction module B for mixing; when the mixed material enters the fourth reaction module C, 184.5g of nitrobenzene in the pre-storage tank V-03 Pump in; through the tube reactor delay pipel...
Embodiment 2
[0039] 1) After the two reaction modules A1 and A2 are connected in parallel, they are connected in series with the two reaction modules B and C in sequence, and the microchannel reactor is replaced with a protonic acid; 106g (1mol) of benzaldehyde and 67g (1mol) of pyrrole are weighed and mixed into a homogeneous solution Put it into storage tank V-01, with a volume of about 170mL; weigh 727g of acetic acid and put it into storage tank V-02, with a volume of about 693mL;
[0040] 2) Control the volume flow rate of the mixed solution of benzaldehyde and pyrrole to 2.0mL / min, enter the first reaction module A1 by pump P-01 to preheat 120°C; control the volume flow rate of acetic acid to 8.2mL / min by pump P-02 Enter the second reaction module A2 to preheat 115°C; then enter the third reaction module B for mixing; when the mixed material enters the fourth reaction module C, the compressed air is fed at 80cm3 / min; through the tube reaction The delay pipeline of the device is compl...
Embodiment 3
[0045] 1) After the two reaction modules A1 and A2 are connected in parallel, they are connected in series with the two reaction modules B and C in sequence, and the microchannel reactor is replaced with a protonic acid; 106g (1mol) of benzaldehyde and 67g (1mol) of pyrrole are weighed and mixed into a homogeneous solution Put it into storage tank V-01, with a volume of about 170mL; weigh 788g of acetic anhydride and put it into storage tank V-02, with a volume of about 730mL;
[0046] 2) Control the volume flow rate of the mixed solution of benzaldehyde and pyrrole to 2.0mL / min and enter the first reaction module A1 to preheat at 120°C through the pump P-01; control the volume flow rate of acetic anhydride to 9.2mL / min from the pump P- 02 Enter the second reaction module A2 to preheat at 115°C; then enter the third reaction module B for mixing; when the mixed material enters the fourth reaction module C, the compressed air is fed at 30cm3 / min; through the tube Reactor delay p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com