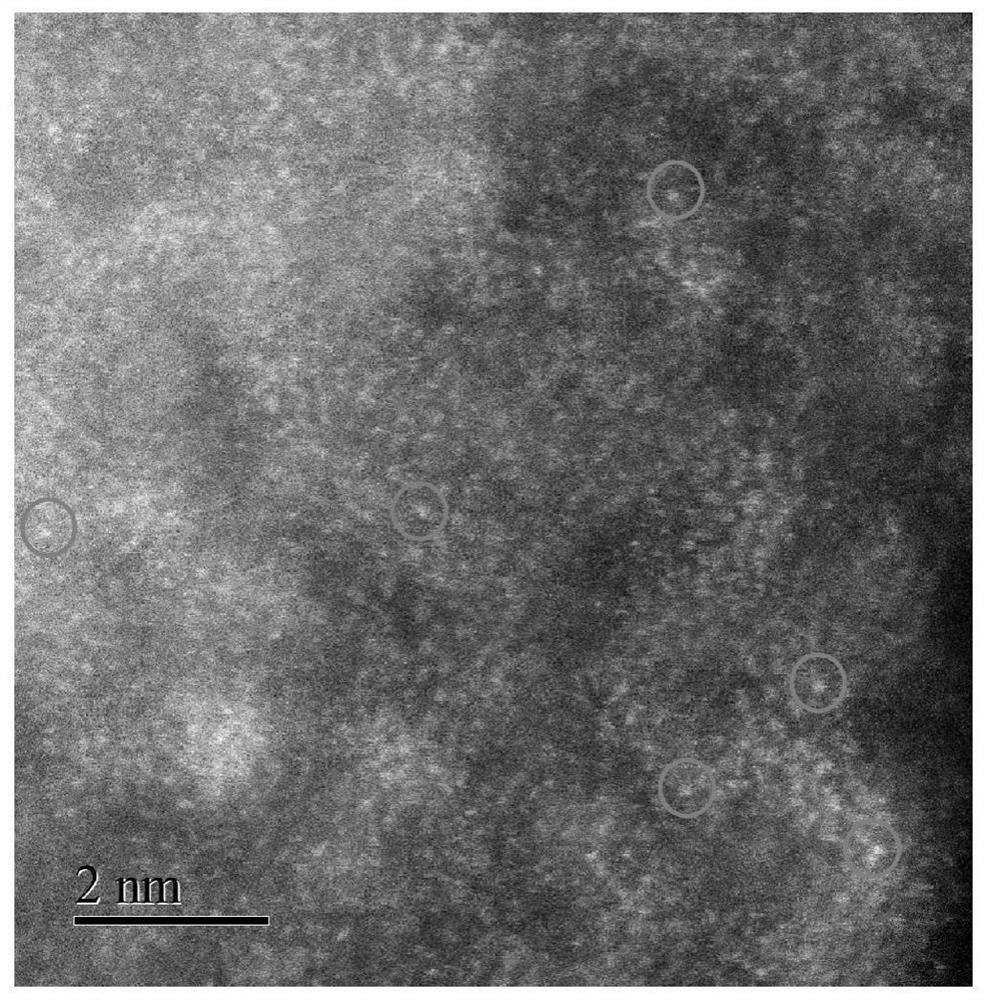
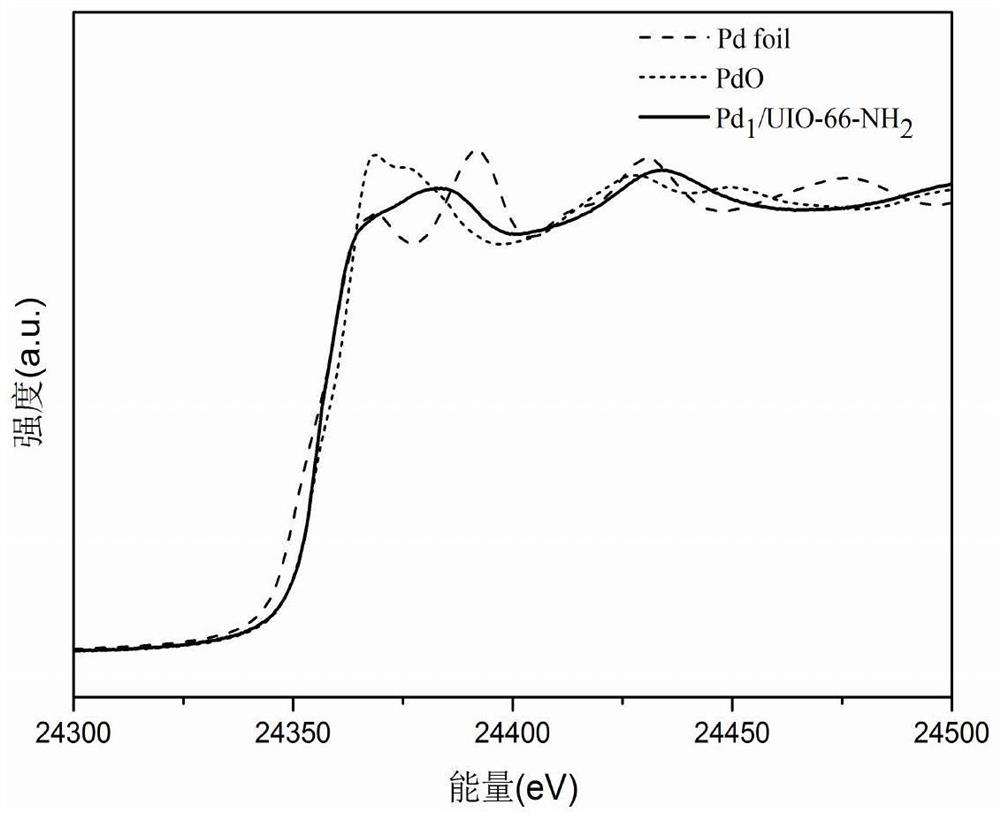
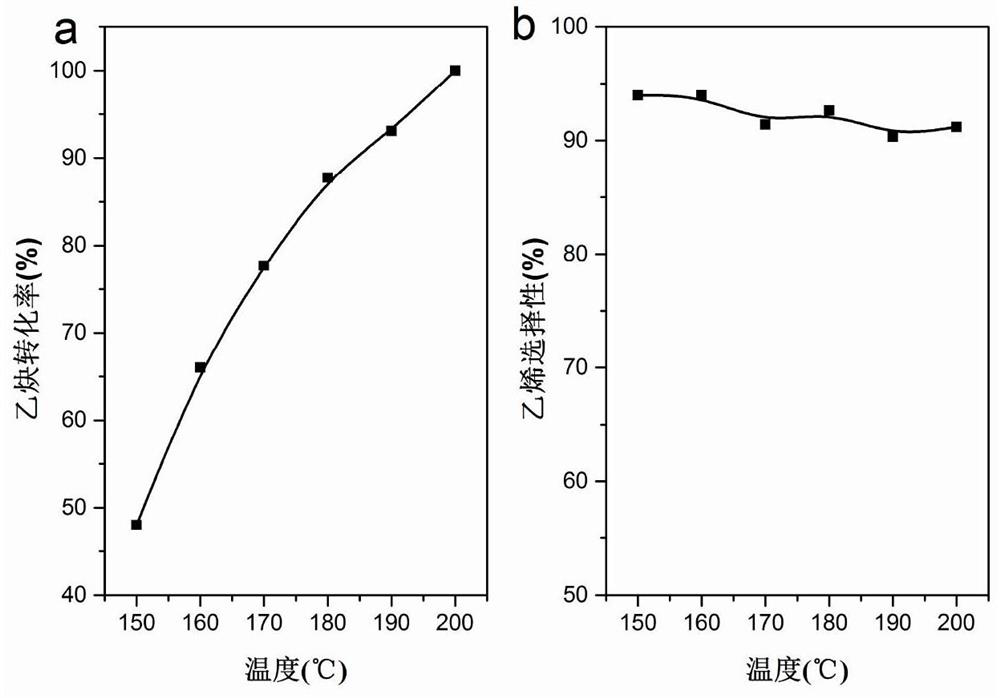
MOF-based transition metal monatomic catalyst for carbon-carbon triple bond selective hydrogenation and preparation method of MOF-based transition metal monatomic catalyst
A transition metal, selective technology, applied in catalyst activation/preparation, physical/chemical process catalysts, hydrogenation to hydrocarbons, etc., can solve the problems of easy agglomeration coupling, no relevant reports, affecting catalytic activity and stability, etc. Achieve the effect of outstanding catalytic performance, simple process, easy recovery and reuse
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A. 1.18g soluble ZrCl 4 and 0.9g of 2-aminoterephthalic acid were dissolved in 60mLN, N-dimethylformamide for ultrasonic dissolution;
[0036] B. Uniformly mix the mixed solution in step A with the glacial acetic acid solution at a volume ratio of 6:1, transfer to a reaction kettle, crystallize at 120°C for 24 hours, cool to room temperature naturally, filter the precipitate, and use N, N-dimethylformamide solution and absolute ethanol were washed until the pH value was neutral, and the solid was dried at 60 °C for 12 h to obtain a metal-organic framework material with an average pore size of 1.0 nm, expressed as UiO-66-NH 2 ;
[0037] C. The soluble transition metal salt PdCl 2 Soluble in deionized water to make Na with a concentration of 50mmol / L 2 PdCl 4 solution; take 1mL Na 2 PdCl 4 The solution was added to anhydrous methanol containing 2-pyridinecarbaldehyde, and stirred continuously at room temperature for 1 h;
[0038] D. At room temperature, according t...
Embodiment 2
[0043] A, B are with embodiment 1;
[0044] C. The soluble transition metal salt PdCl 2 Soluble in deionized water to make Na with a concentration of 50mmol / L 2 PdCl 4 solution; take 1mL Na 2 PdCl 4 The solution was added to anhydrous methanol containing 2-pyridinecarbaldehyde, and stirred continuously at room temperature for 1 h;
[0045] D. At room temperature, according to the fact that the transition metal accounts for 0.1wt.% of the mass of the carrier, fully disperse the carrier in step B into the impregnation solution of step C, keep stirring for 72h, filter, and wash with anhydrous methanol and dichloroethane , and dried at 60°C for 12 hours to obtain Pd 1 / UiO-66-NH 2 ;
[0046] E. Pd obtained in step D 1 / UiO-66-NH 2 Placed in the reactor, passing H 2 / C 2 h 2 / C2 h 4 atmosphere, wherein the molar ratio of alkynes to alkenes is 0.01, alkynes and H 2 The molar ratio is 0, the flow rate is 10mL / min, and at 10℃·min -1 The temperature is raised to 150 ° C ...
Embodiment 3
[0048] A. 1.18g soluble ZrCl 4 and 1.28g of 2-amino-4,4'-biphenyldicarboxylic acid were dissolved in 60mL of N,N-dimethylformamide for ultrasonic dissolution;
[0049] B. Uniformly mix the mixed solution in step A with the glacial acetic acid solution at a volume ratio of 6:1, transfer to a reaction kettle, crystallize at 120°C for 24 hours, cool to room temperature naturally, filter the precipitate, and use N, N-dimethylformamide solution and absolute ethanol were washed until the pH value was neutral, and the solid was dried at 60 °C for 12 h to obtain a metal-organic framework material with an average pore size of 1.5 nm, expressed as UiO-67-NH 2 ;
[0050] C. The soluble transition metal salt PtCl 2 Soluble in deionized water to make Na with a concentration of 50mmol / L 2 PtCl 4 solution; take 1mL Na 2 PtCl 4 The solution was added to anhydrous methanol containing 2-pyridinecarbaldehyde, and stirred continuously at room temperature for 1 h;
[0051] D. At room temper...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com