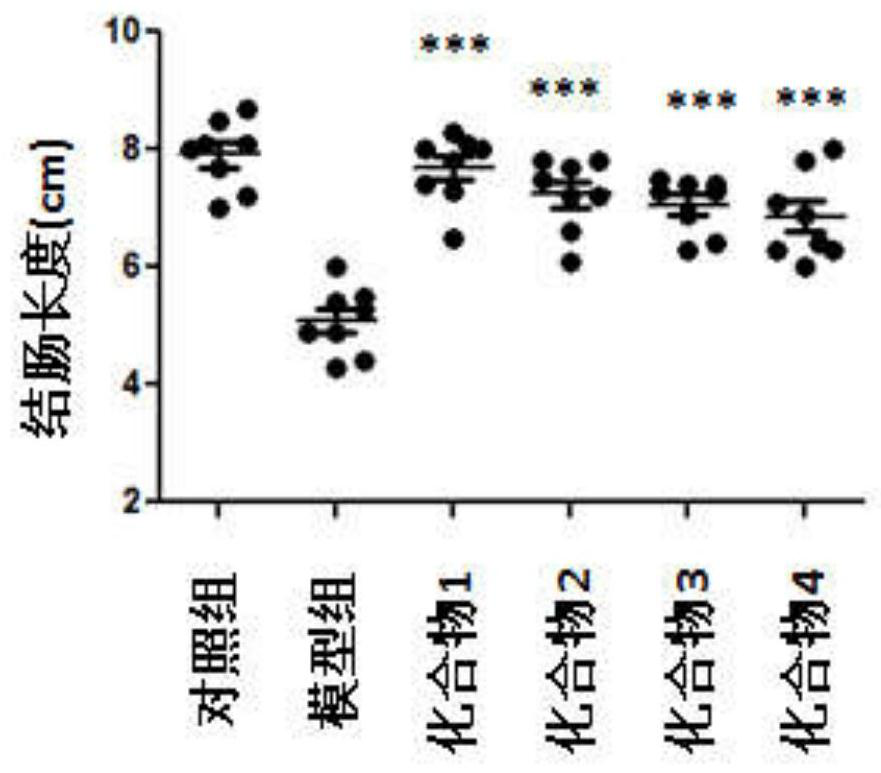
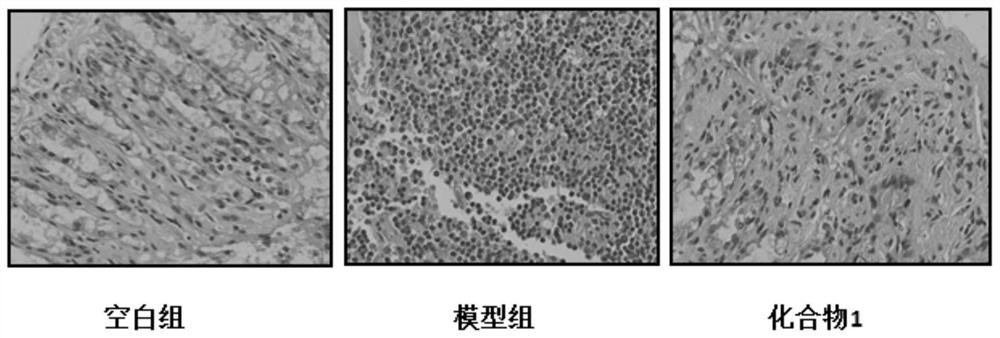
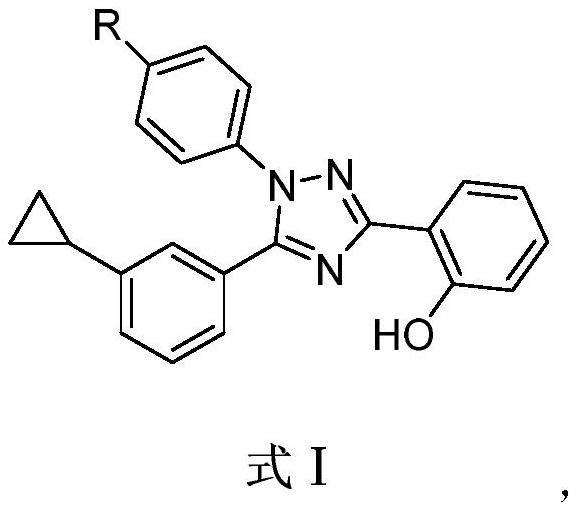
1, 2, 4-triazole derivative compound and application thereof in treating inflammatory bowel disease
A technology of derivatives and compounds, applied in the direction of drug combinations, anti-inflammatory agents, digestive system, etc., can solve problems that have not been reported, achieve good preventive or therapeutic effects, and good development prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1, the preparation of compound 1
[0039] (1), the synthesis of 3-cyclopropyl benzimidic acid methyl ester
[0040]
[0041] Dissolve 3-cyclopropylbenzonitrile (150mmol) in methanol (150mL), cool down to 0°C, then pass HCl gas for about 4 to 5 hours, react overnight at constant temperature, concentrate, and add the obtained solid to diethyl ether for beating to obtain a white Solid 3-cyclopropylbenzimidic acid methyl ester hydrochloride, then the above white solid was added to water (250mL), cooled to 10-20°C, and solid sodium bicarbonate (130mmol) was added in batches to obtain a mixture Dichloromethane (200mL×3) was added to extract, the organic layers were combined, dried by adding anhydrous sodium sulfate, filtered, and the filtrate was concentrated to obtain methyl 3-cyclopropylbenzimidate, 22.6g, with a yield of 86.0%.
[0042] 1 H-NMR (400MHz, CDCl 3 )δ:1.33(m,2H),1.51(m,2H),2.74(m,1H),3.84(s,3H),7.19-7.28(m,2H),7.64(m,1H),7.81(m ,1H),9.13(s,1H)...
Embodiment 2
[0054] Embodiment 2, the preparation of compound 2
[0055]
[0056] 2-(1-(4-chlorophenyl)-5-(3-cyclopropylphenyl)-1H-1,2,4-triazol-3-yl)phenol (50mmol) in absolute ethanol ( 100ml) solution was stirred thoroughly, then 2-(piperazin-1-yl)ethan-1-ol (120mmol) was added, and after stirring at room temperature for 10-12 hours, petroleum ether (150ml) was added to crystallize the product and filtered. Subsequent purification using flash column chromatography gave off-white solid 2-(5-(3-cyclopropylphenyl)-1-(4-(4-(hydroxymethyl)piperidin-1-yl)phenyl)- 1H-1,2,4-triazol-3-yl)phenol (compound 2), 18.2 g, yield 78.0%. LC-MS (ESI, pos, ion) m / z: 467 [M+H].
Embodiment 3
[0057] Embodiment 3, the preparation of compound 3
[0058]
[0059]2-(1-(4-chlorophenyl)-5-(3-cyclopropylphenyl)-1H-1,2,4-triazol-3-yl)phenol (50mmol) in absolute ethanol ( 100ml) solution was stirred well, then 2-(piperazin-1-yl)ethan-1-ol (120mmol) was added, and after stirring at room temperature for 10-12 hours, petroleum ether (150ml) was added to crystallize the product and filtered. Subsequent purification using flash column chromatography afforded 2-(5-(3-cyclopropylphenyl)-1-(4-(4-propylpiperidin-1-yl)phenyl)-1H-1 as an off-white solid ,2,4-triazol-3-yl)phenol (compound 3), 17.0 g, yield 71.0%. LC-MS (ESI, pos, ion) m / z: 479 [M+H].
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com