Label-free human galectin 13 as well as preparation method and application thereof
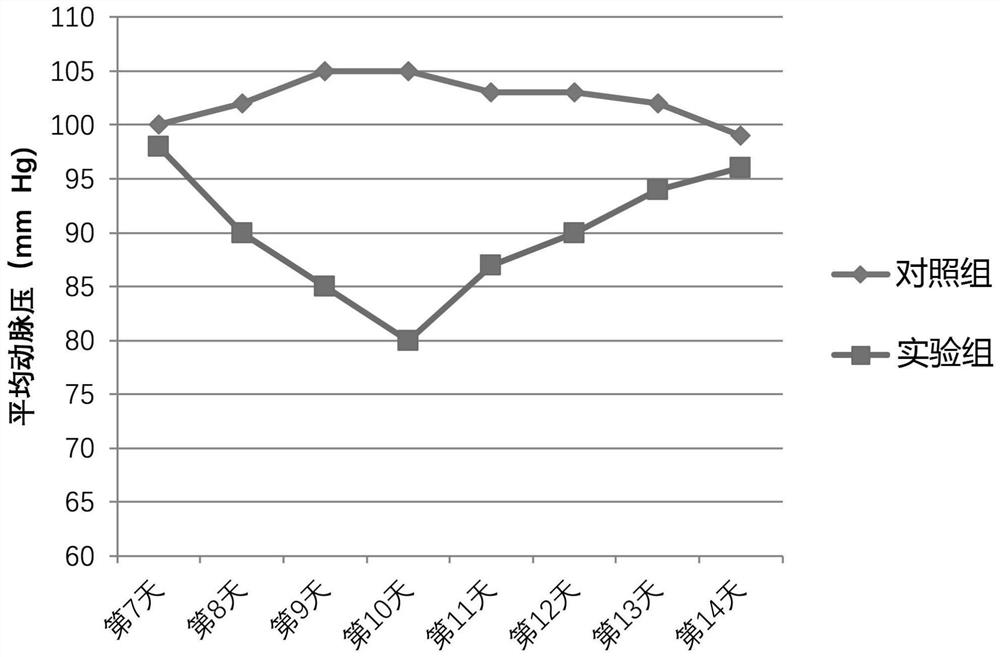
A galectin, label-free technology, applied in the field of label-free human galectin 13 and its preparation, to achieve the effect of lowering blood pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] The preparation method of the unlabeled human galectin-13 of the present invention specifically comprises the following steps:
[0041]Step 1. Insert the human galectin-13 gene into the plasmid pET15b, the restriction endonuclease sites are NcoI and XhoI or BamHI, and the end of the human galectin-13 cDNA is added with a stop codon TAA, The obtained plasmid was named pET15b_Galectin-13;
[0042] Among them, the gene of human galectin 13 is derived from human (Homo sapiens) or derived from gene synthesis.
[0043] Among them, the gene of human galectin 13 is cDNA or a synthetic gene.
[0044] Step 2, transforming the plasmid pET15b_Galectin-13 into Escherichia coli BL21, Escherichia coli BL21(DE3) or Escherichia coli BL21(DE3)pLysS;
[0045] Step 3: Pick a number of Escherichia coli colonies and place them in LB medium, and culture them in a shaker, the temperature in the shaker is 37°C, and the rotation speed is 150-220rpm; wait for OD 600 To 0.2-4, add 0.1-10mM IPTG...
Embodiment 1
[0073] Example 1 Preparation of unlabeled human galectin 13
[0074] (1) Insert the cDNA of human galectin 13 into the plasmid pET15b, the restriction endonuclease sites are NcoI and XhoI or BamHI, and the end of the cDNA of human galectin 13 is added with a stop codon TAA, The obtained plasmid was named pET15b_Galectin-13;
[0075] (2) Transform the plasmid pET15b_Galectin-13 into Escherichia coli BL21;
[0076] (3) Pick a number of Escherichia coli colonies and place them in LB medium, and place them in a shaker for cultivation. The temperature in the shaker is 37° C., and the rotation speed is 150 rpm; 600 To 0.2-4, add 10mM IPTG to induce protein expression, and the induction time is 24h; the bacteria are collected by centrifugation at 6000g;
[0077] (4) Add bacterial lysate (10mM Hepes, pH7.0) and ultrasonically lyse for 30min;
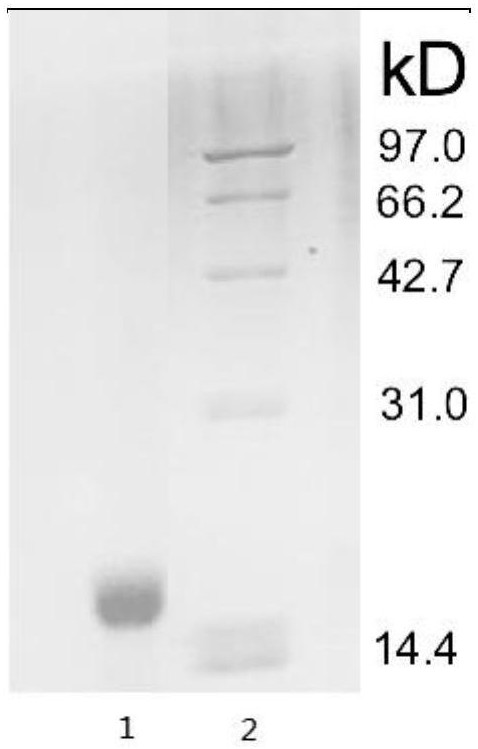
[0078] (5) Centrifuge at 12000g to remove the precipitate, collect the supernatant, which contains unlabeled human galectin-13, and the oper...
Embodiment 2
[0090] Example 2 Preparation of unlabeled human galectin 13
[0091] (1) Insert the cDNA of human galectin 13 into the plasmid pET15b, the restriction endonuclease sites are NcoI and XhoI or BamHI, and the end of the cDNA of human galectin 13 is added with a stop codon TAA, The obtained plasmid was named pET15b_Galectin-13;
[0092] (2) Transform the plasmid pET15b_Galectin-13 into Escherichia coli BL21 (DE3);
[0093] (3) Pick a number of Escherichia coli colonies and place them in LB medium, place them in a shaker for cultivation, the temperature in the shaker is 37°C, and the rotation speed is 220rpm; 600 To 0.2-4, add 0.1mM IPTG to induce protein expression, the induction time is 2h; 5000g centrifuge to collect bacteria;
[0094] (4) Add bacterial lysate (100mM Hepes, pH7.0) and ultrasonically lyse for 2min;
[0095] (5) Centrifuge at 8000g to remove the precipitate, collect the supernatant, which contains unlabeled human galectin-13, and the operating temperature is 4°...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com