Preparation method of p-fluorobenzaldehyde
A technology of p-fluorobenzaldehyde and fluorobenzene, which is applied in the field of preparation of p-fluorobenzaldehyde, can solve the problems of high process temperature and pressure, high equipment requirements, and many solid wastes, and achieve low equipment requirements, reduce production costs, The effect of avoiding security risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
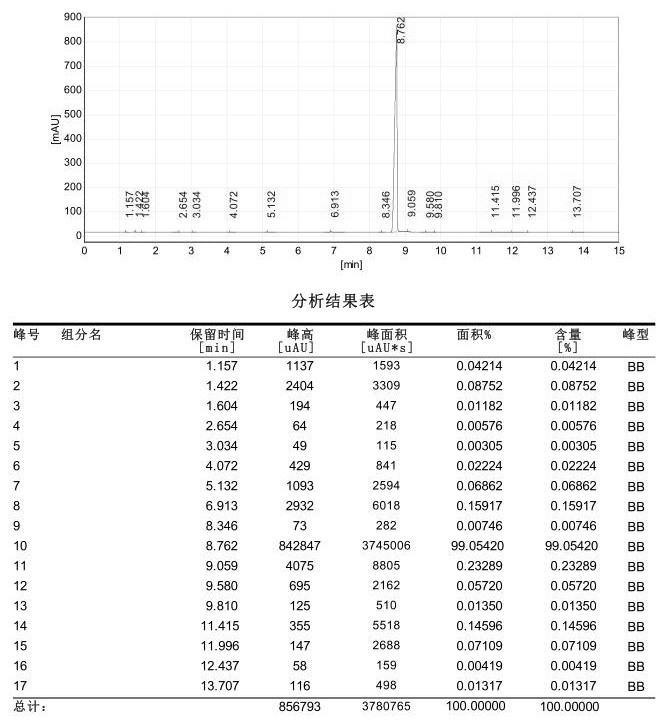
Image
Examples
Embodiment 1
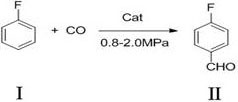
[0018] A preparation method of p-fluorobenzaldehyde, the synthetic route of p-fluorobenzaldehyde (II) is as follows:
[0019]
[0020] A preparation method for p-fluorobenzaldehyde, comprising the following steps:
[0021] Add fluorobenzene (I), catalyst anhydrous aluminum chloride and co-catalyst into the reaction kettle, seal it, pressurize it with nitrogen and carbon monoxide to 1.0kg respectively, and replace it, and then introduce carbon monoxide to control the temperature at 30°C and the pressure at 2.0MPa React until carbon monoxide is no longer inhaled. After the reaction is completed, release the pressure to normal pressure. Control 30°C with 10% hydrochloric acid aqueous solution for acid hydrolysis, then wash with water until neutral, and vertical oil-free vacuum precipitation to an internal temperature of 100 Stop at ℃, and rectify to get p-fluorobenzaldehyde (Ⅱ).
[0022] The mass fraction ratio of fluorobenzene (I) to the catalyst is 5:1; the cocatalyst is a ...
Embodiment 2
[0024] A preparation method of p-fluorobenzaldehyde, the synthetic route of p-fluorobenzaldehyde (II) is as follows:
[0025]
[0026] A preparation method for p-fluorobenzaldehyde, comprising the following steps:
[0027] Add fluorobenzene (I), catalyst anhydrous aluminum chloride and co-catalyst to the reaction kettle, seal it, press it with nitrogen and carbon monoxide to 1.0kg respectively, and then replace it, and then introduce carbon monoxide to control the temperature at 40°C and the pressure at 0.8MPa to react Until the carbon monoxide is no longer inhaled, after the reaction is completed, the pressure is released to normal pressure, under the control of 20°C, acid hydrolysis is carried out with 10% hydrochloric acid aqueous solution by mass percentage, then washed with water until neutral, and the vertical oil-free decompression precipitation is carried out to the internal temperature Stop at 100°C, and obtain p-fluorobenzaldehyde (Ⅱ) through rectification.
[00...
Embodiment 3
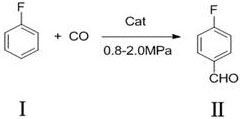
[0030] A preparation method of p-fluorobenzaldehyde, the synthetic route of p-fluorobenzaldehyde (II) is as follows:
[0031]
[0032] A preparation method for p-fluorobenzaldehyde, comprising the following steps:
[0033] Add fluorobenzene (I), catalyst anhydrous aluminum chloride and co-catalyst into the reaction kettle, seal it, pressurize it with nitrogen and carbon monoxide to 1.0kg respectively, and then replace it, and then introduce carbon monoxide to control the temperature at 50°C and the pressure at 1.2MPa React until carbon monoxide is no longer inhaled. After the reaction is completed, release the pressure to normal pressure. Under the control of 25°C, use 10% hydrochloric acid aqueous solution for acid hydrolysis, then wash with water until neutral, and vertical oil-free decompression precipitation to the inner Stop when the temperature is 100°C, and rectify to obtain p-fluorobenzaldehyde (Ⅱ).
[0034] The mass fraction ratio of fluorobenzene (I) to the catal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com