Chiral resolution method of isobutyrate compound
A technology of chiral resolution and isobutyrate, which is applied in fermentation and other directions, can solve the problems of high cost and complicated operation of chiral resolution, and achieve the effect of easy availability of raw materials and catalysts, simple operation and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
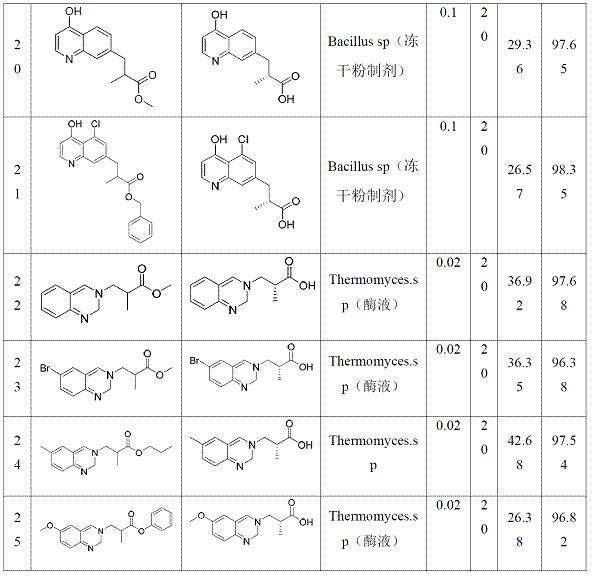
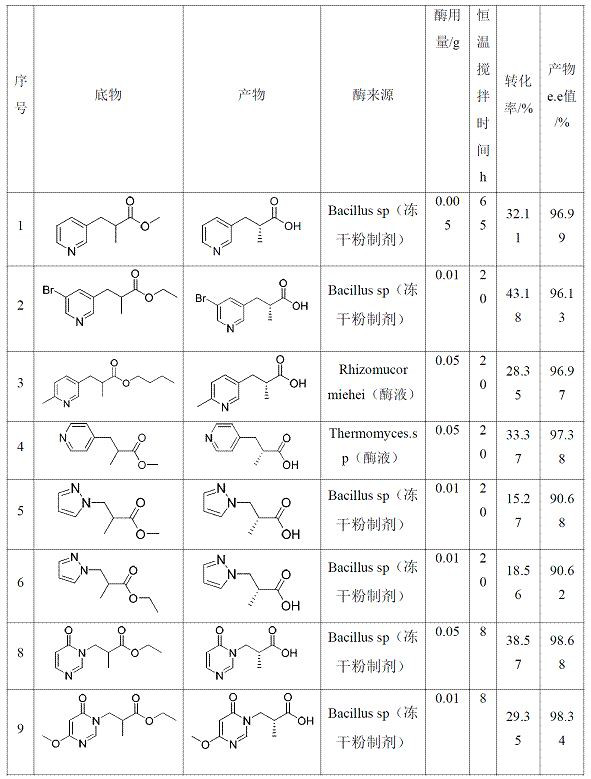
[0041] In a 10 mL reaction bottle, add 0.1 g of substrate ester into 5 mL of 0.3 M potassium phosphate buffer solution, adjust the pH to 7.0~7.5, add 0.005~0.1 g of hydrolase (lyophilized powder preparation or enzyme solution, see Table 1) Form a hydrolysis reaction system, control the pH of the hydrolysis reaction system to 7.0~7.5, stir at a constant temperature of 30°C±3°C for a certain period of time, and obtain a hydrolyzed system. Take 100 μL of the hydrolyzed system and stop the reaction with 900 μL of 50% acetonitrile, shake and mix well, and centrifuge at 8000 rpm for 1 min to obtain the supernatant aqueous phase and organic solid phase, which are dried with nitrogen, dissolved in ethanol, and detected by HPLC According to the efficiency and chirality, 100 kinds of hydrolases were screened for the following substrates (these 100 kinds of hydrolases are all from commercial enzymes, artificially synthesized from known sequences reported in literature or obtained by artif...
Embodiment 2
[0047] (1) Feeding: Add 0.5 g of hydrolase Thermomyces.sp, 30 mL of tris-hydrochloric acid buffer (300 mM, pH=9.0) into a 100 mL reaction bottle, stir, and dissolve the enzyme in tris In aminomethane-hydrochloric acid buffer;
[0048] (2) Substrate addition: add 1 g of the main raw material to the above reaction bottle , stirring, the system pH=9.0;
[0049] (3) Reaction: The system was reacted at 30°C, stirred for 24 hours, and the pH of the system was maintained to 9.0 with 1N NaOH;
[0050] (4) Post-treatment: Add 20 mL of acetonitrile to the system after the reaction, stir for 30 minutes, and then filter through a diatomaceous earth pad. Adjust the pH of the obtained organic phase system to 8~9 with sodium bicarbonate solution, add 20 mL of ethyl acetate for extraction three times, and water The pH of the phase was adjusted to 2~3 with 0.5 M hydrochloric acid, and the product was precipitated, and the temperature of the product was controlled at 40~45°C to concentrate a...
Embodiment 3
[0052] (1) Feeding: Add 0.05 g of hydrolase Thermomyces.sp, 25 mL of potassium phosphate buffer (100 mM, pH=7.5) into a 100 mL reaction bottle, stir, and dissolve the enzyme in potassium phosphate buffer; add 2.5 mL ethyl acetate
[0053] (2) Substrate addition: add 1 g of the main raw material to the above reaction flask , stirred, and maintained the pH of the system to 7.5 with 1N NaOH;
[0054] (3) Reaction: The system was reacted at 40°C, stirred for 120 hours, and the pH of the system was maintained to 7.5 with 1N NaOH;
[0055] (4) Post-treatment: Take 100 μL of the system and stop the reaction with 900 μL of 50% acetonitrile. After fully shaking and mixing, centrifuge at 8,000 rpm for 1 min. After drying the supernatant liquid phase with nitrogen, dissolve it in ethanol, and detect the conversion rate by HPLC. is 45.67%, and the product is The e.e. value was 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com