ORR catalyst material and preparation method and application thereof
A catalyst and carbon material technology, applied in the field of catalyst materials, can solve the problems of large amount of precious metals, small specific surface area, high cost, etc., and achieve the effect of simple preparation method, high specific surface area and high porosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
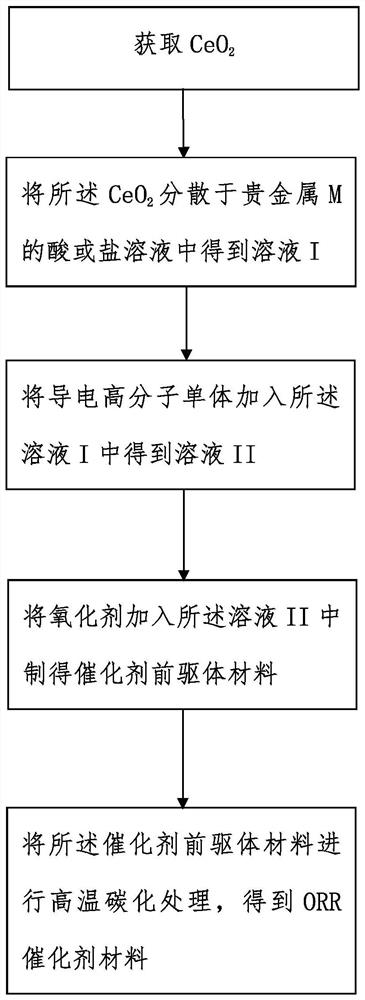
[0065] see figure 1 , a preparation method of the aforementioned ORR catalyst material, comprising the following steps:
[0066] 1) Get CeO 2 ;
[0067] 2) Utilize the CeO 2 A catalyst precursor material is prepared, the catalyst precursor material is a conductive polymer composite material doped with noble metal acid groups, and the chemical formula of the catalyst precursor material is CeO 2 / CP-MX n- , X is an acid radical, n is 1 or 2;
[0068] 3) Carbonizing the catalyst precursor material at high temperature to prepare the ORR catalyst material.
[0069] The step 1) obtains Ce0 2, , commercially available micron and / or nanoscale Ce0 2 , which can also be prepared as follows:
[0070] 1.1) dissolving cerium nitrate and sodium hydroxide in deionized water, adjusting the pH value of the solution, and stirring to obtain a reaction solution; preferably, in the step 1.1), the molar ratio of the cerium nitrate and the sodium hydroxide is 1:4, the pH value is 12, and th...
Embodiment 1
[0090] Prepare a cerium nitrate solution with a concentration of 0.1M and adjust it to pH = 12 with sodium hydroxide. After magnetically stirring for 2 hours, place the solution in an oven at 60°C for 1 hour. After filtering and washing, place the yellow solid in an oven at 130°C. After annealing for 4h, cerium oxide was obtained after cooling to room temperature for 2h.
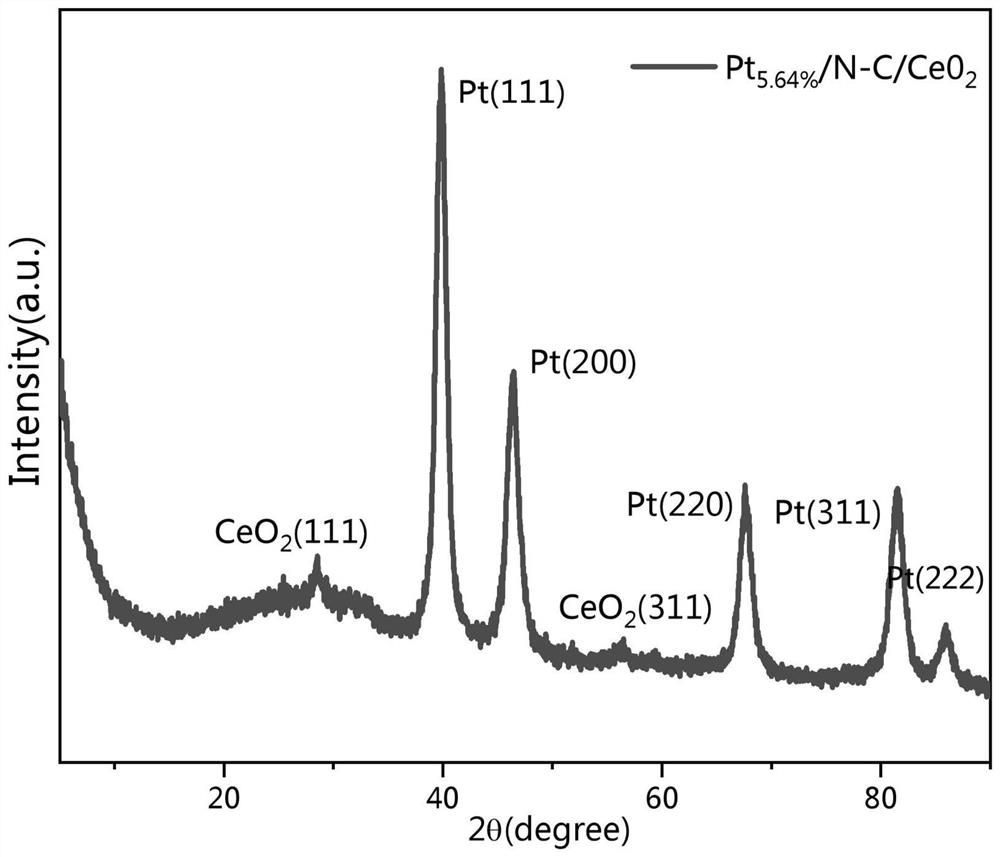
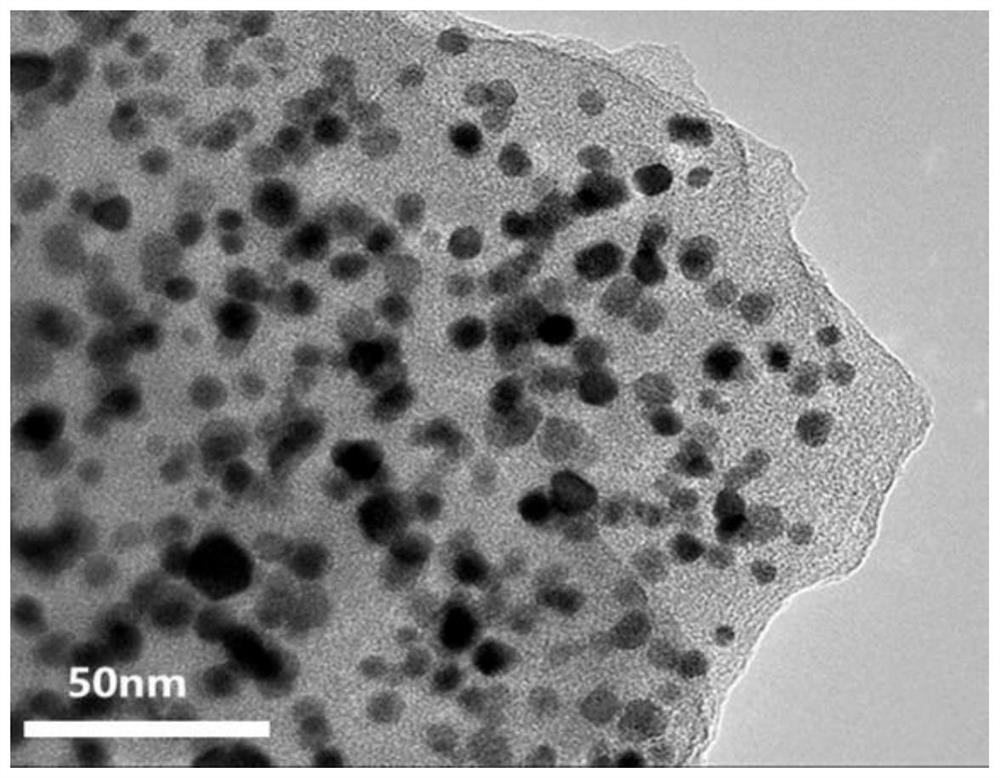
[0091] With 0.6g (3.5mmol) cerium oxide, 0.1g (0.2mmol) potassium chloroplatinate (K 2 PtCl 6 ) was added to 40ml of 1mol / L HCl solution to obtain solution I. After ultrasonic dispersion for 2 hours, 0.6ml of aniline monomer (6.4mmol) was added to solution I to obtain solution II; 0.6g of APS (4mmol) was dissolved in 10ml of 1mol / LHCl solution to obtain solution III, and disperse it evenly by ultrasonication for 2 hours; control the dropping speed of solution III so that it slowly drops into solution II; keep at 5°C and react for 5 hours. The reaction process is accompanied by magnetic stirring; the catal...
Embodiment 2
[0099] Embodiment 2 (difference from embodiment 1 is that potassium chloroplatinate consumption is 0.05g)
[0100] Prepare a cerium nitrate solution with a concentration of 0.1M and adjust it to pH = 12 with sodium hydroxide. After magnetically stirring for 2 hours, place the solution in an oven at 60°C for 1 hour. After filtering and washing, place the yellow solid in an oven at 130°C. After annealing for 4h, cerium oxide was obtained after cooling to room temperature for 2h.
[0101] With 0.6g (3.5mmol mol) cerium oxide, 0.05g (0.1mmol) potassium chloroplatinate (K 2 PtCl 6 ) was added into 40ml of 1mol / L HCl solution to obtain solution I. After ultrasonic dispersion for 2 hours, 0.6ml (6.4mmol) aniline monomer was added to solution I to obtain solution II; 0.6g (4mmol) APS was dissolved in 10ml of 1mol / LHCl solution to obtain solution III, ultrasonically for 2 hours to disperse evenly; control the dripping speed of solution III to slowly drip into solution II; keep at 5°...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com