Application of fluorine-substituted 2-aminothiazole-5-aromatic carboxamide
A technology of usage and metabolites, applied in the field of medicinal chemistry, can solve problems such as loss of activity, mutation of target protein, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
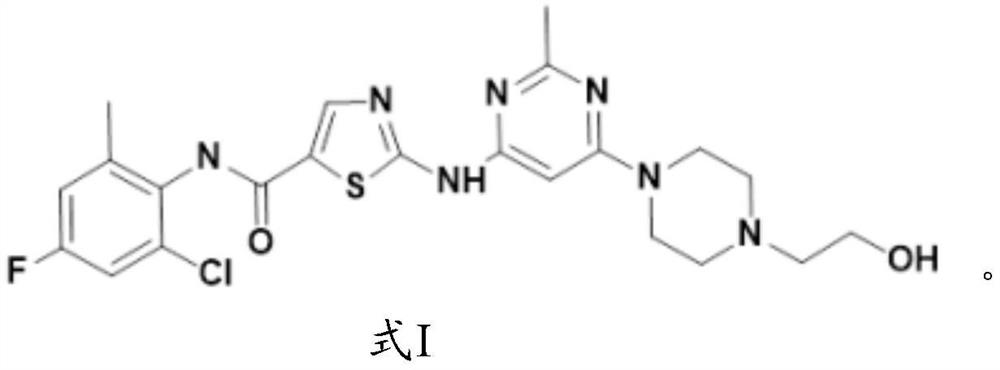
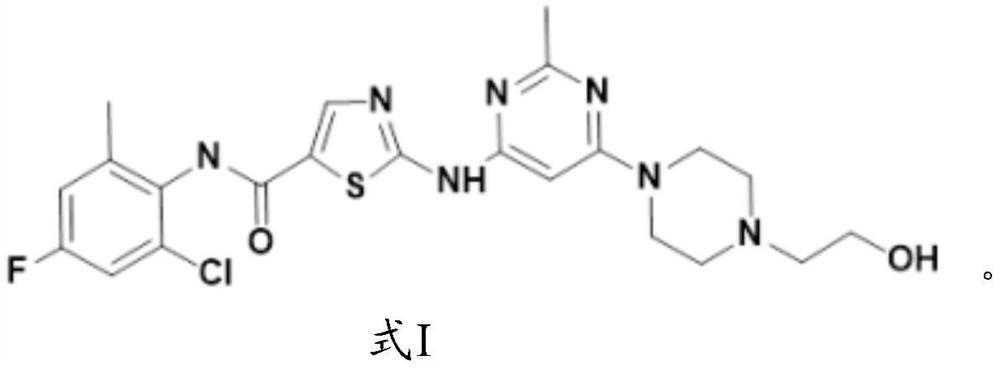
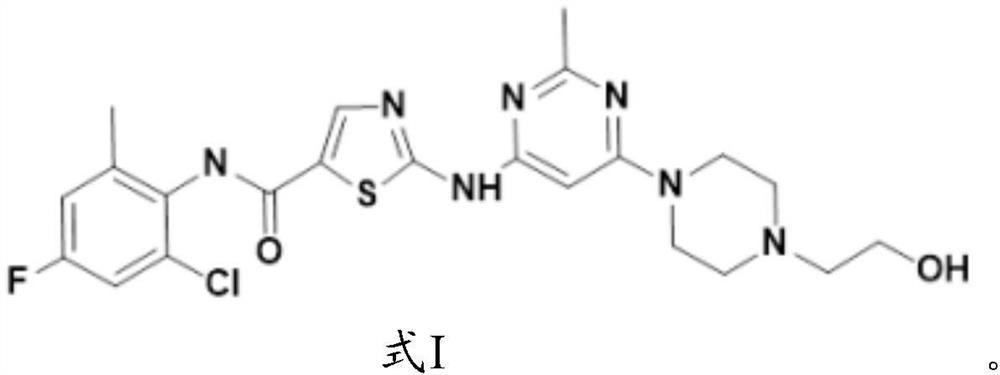
[0081] The synthesis of formula I compound:
[0082]
[0083] The synthetic route of formula I compound is as follows:
[0084] Synthesis of [5-(2-chloro-4-fluoro-6-methylphenylcarbamoyl)thiazol-2-yl]-carbamic acid tert-butyl ester
[0085]
[0086] N 2 Under gas protection, add 24.4g (0.1mol) of 2-tert-butoxycarbonylaminothiazole-5-carboxylic acid and 0.5ml of DMF (N,N-dimethylformamide) into 250ml of dichloromethane, and slowly add 13ml of Oxalyl chloride solution (0.15mol), reacted for 2h, and removed the solvent by rotary evaporation to obtain a white solid, which was dissolved in 100ml of anhydrous dichloromethane, and slowly added dropwise to 2-chloro-4-fluoro- In the methylene chloride solution of 17.5g (0.11mol) of 6-methylaniline (0.11mol) and N,N-diisopropylethylamine 38.8g (0.3mol), N 2 React at room temperature for 10 h under gas protection, distill off the solvent under reduced pressure, add a mixed solvent of 25 ml of ethyl acetate and 25 ml of n-hexane ...
Embodiment 1
[0097] The compound of formula I was used to study the activity of kinase inhibition in vitro and the metabolism of human liver microsomes.
[0098] 1. Kinase inhibitory activity study
[0099] 1.1 Experimental method
[0100] 1.1.1 Test drug preparation
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com