Improved synthesis process of antihypertensive efonidipine hydrochloride
A technology of efodipine hydrochloride and process, which is applied in the field of synthetic improvement process of antihypertensive ifodipine hydrochloride, can solve the problems of affecting reaction yield, increasing reaction cost, and complicated experimental operation, so as to improve reaction yield and reduce reaction Cost, effect of simplifying experimental operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0037]In order to enable those skilled in the art to better understand the technical solutions of the present invention, the present invention will be described in detail below with reference to the accompanying drawings. The description in this section is only exemplary and explanatory, and should not have any restrictive effect on the protection scope of the present invention. .
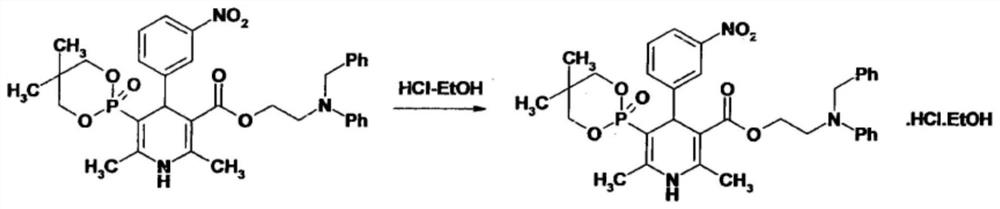
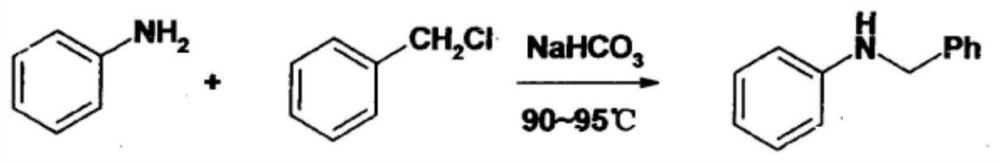
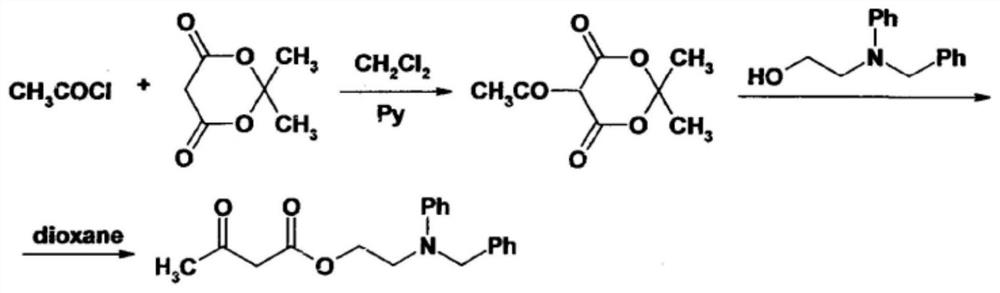
[0038]SeeFigure 1-4, An improved synthesis process of anti-blood pressure ifodipine hydrochloride, including the following steps:
[0039]A. Synthesis of N-benzylaniline: Add 37.23g (0.4mol) aniline, 10.10g (0.12mol) sodium bicarbonate and 100mL distilled water into a 250mL three-necked flask, heat to 90~95℃ with stirring, and wait for the temperature When it is constant, use a constant pressure dropping funnel to slowly drop 12.65g (0.1mol) of benzyl chloride into a three-necked flask. TLC (petroleum ether / ethyl acetate=20 / 1) monitors the reaction. After 2 hours, the reaction is complete and the reaction is s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com