a 2 mn 2-x w x o 6 Composite catalytic material of perovskite oxide and nitrogen-doped carbon, preparation method and application thereof
A technology of perovskite oxides and catalytic materials, which is applied in fuel cell-type half-cells and primary battery-type half-cells, structural parts, electrical components, etc. In order to achieve the effect of improving the reaction efficiency and the overall performance of the battery, good conductivity and dispersibility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1.1 Preparation of Ca 2 Mn 1.2 W 0.8 O 6 Perovskite Oxides:
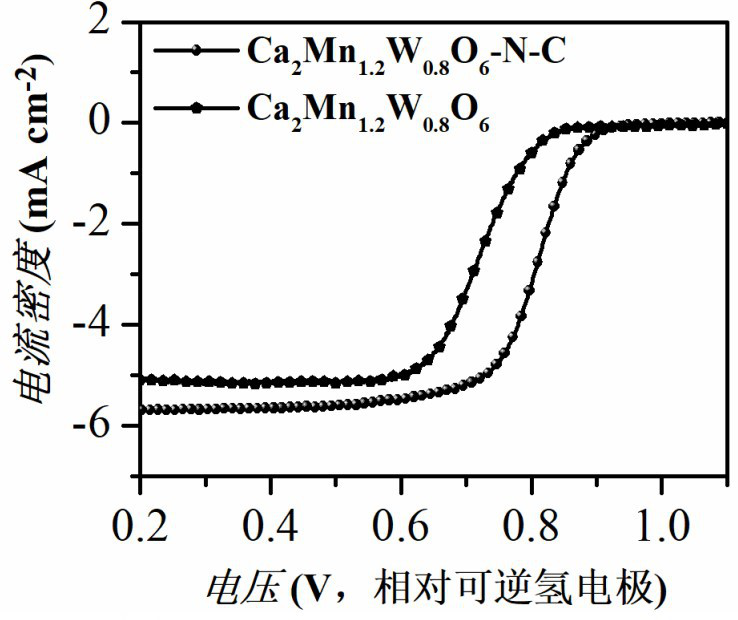
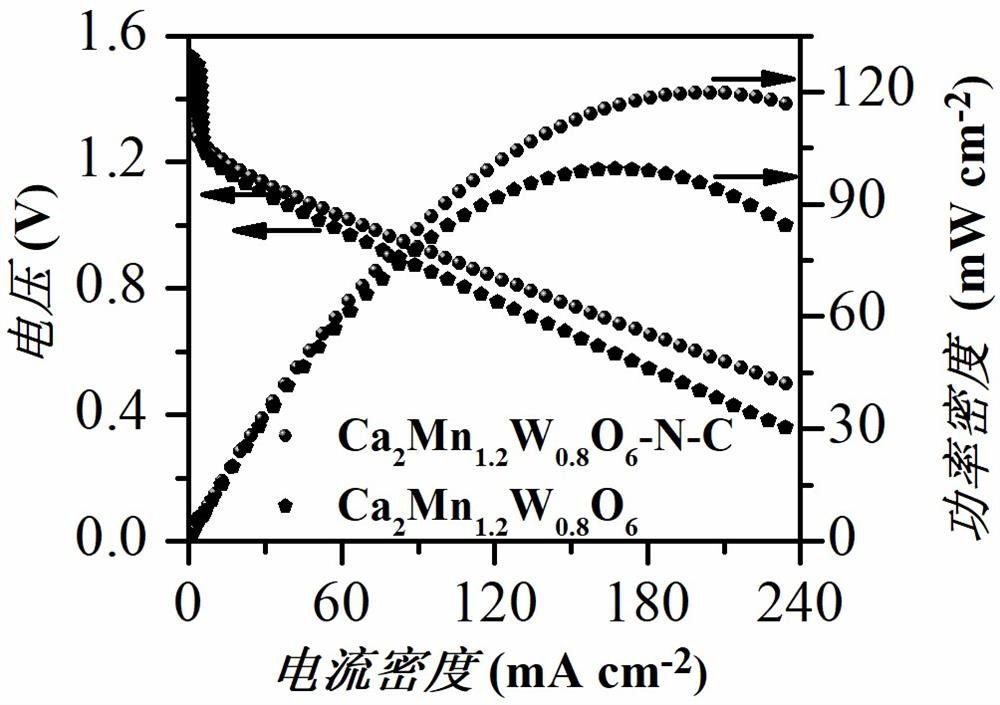
[0026] Calcium nitrate, manganese acetate tetrahydrate, and ammonium metatungstate are dissolved in deionized water in a stoichiometric ratio, and then citric acid monohydrate is added to the above-mentioned metal salt solution, wherein citric acid monohydrate and metal ions (including calcium ions, The molar ratio of manganese ion and tungsten ion) is 1.5:1, then the above solution is heated and stirred continuously in a constant temperature water bath of 80 ° C until a gel is formed, and the obtained gel is dried in a blast drying oven at 140 ° C for 12 hours, The temperature is then raised to 240° C. for 10 hours to obtain a catalyst material precursor. The catalytic material precursor was calcined in a muffle furnace at 1000 °C for 8 hours, and then transferred to a tube furnace and calcined at 1050 °C for 10 hours in a high-purity mixture of hydrogen / nitrogen to obtain Ca 2 Mn 1.2 W 0.8 O 6 Perov...
Embodiment 2
[0036] 2.1 Preparation of Ba 1.6 Sr0.4 MnWO 6 Perovskite Oxides:
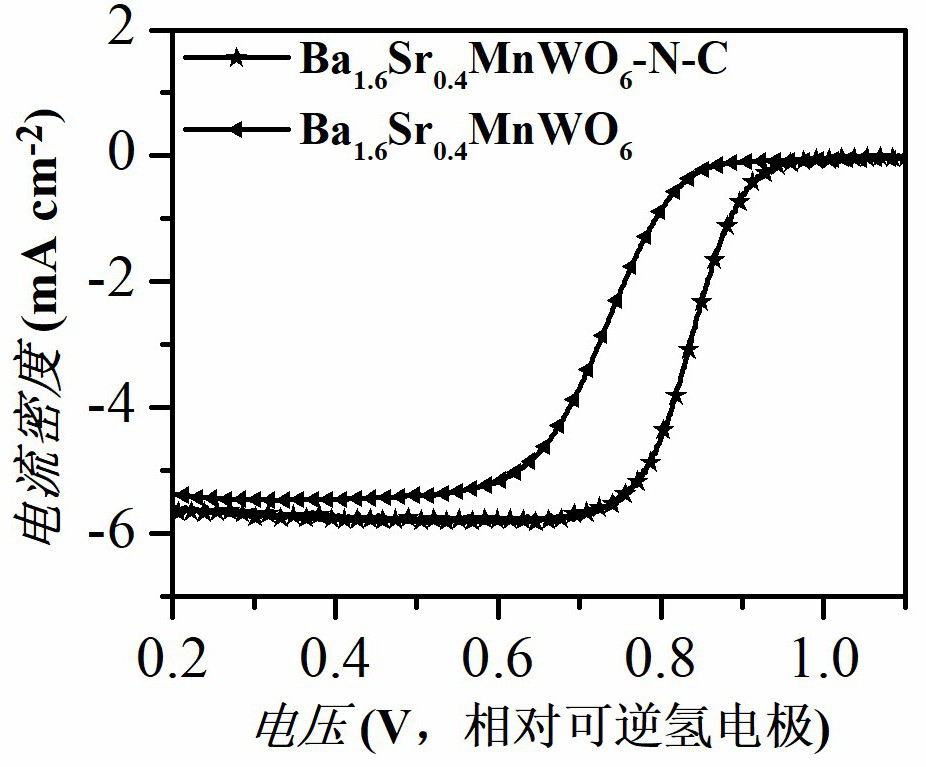
[0037] Dissolve barium nitrate, strontium nitrate, manganese acetate tetrahydrate, and ammonium metatungstate in deionized water at a stoichiometric ratio, and then add citric acid monohydrate to the above metal salt solution, wherein citric acid monohydrate and metal ions (including The molar ratio of barium ion, strontium ion, manganese ion and tungsten ion) is 2:1, and then the above solution is continuously stirred and heated in a 100 ° C constant temperature water bath until a gel is formed, and the resulting gel is dried in a blast drying oven at 90 ° C. After drying at low temperature for 24 hours, the temperature was raised to 300° C. for 6 hours to obtain a catalyst material precursor. The catalytic material precursor was calcined in a muffle furnace at 800 °C for 12 hours, then transferred to a tube furnace and calcined in high-purity hydrogen at 1100 °C for 15 hours to obtain Ba 1.6 Sr 0.4 MnWO ...
Embodiment 3
[0047] 3.1 Preparation of Sr 2 MnWO 6 Perovskite Oxides:
[0048] Strontium acetate, manganese acetate tetrahydrate, and ammonium metatungstate are dissolved in deionized water according to the stoichiometric ratio, and then citric acid monohydrate is added to the above-mentioned metal salt solution, wherein, citric acid monohydrate and metal ions (including strontium ions, The molar ratio of manganese ion and tungsten ion) is 1: 1, then the above solution is continuously stirred and heated in a constant temperature water bath at 80 ° C until a gel is formed, and the obtained gel is dried in a blast drying oven at 100 ° C for 12 hours, and then The temperature was raised to 250° C. for 6 hours to obtain a catalyst material precursor. The catalyst material precursor was calcined in a muffle furnace at 900 °C for 12 hours, and then transferred to a tube furnace and calcined at 1000 °C for 24 hours in a high-purity mixture of hydrogen / nitrogen to obtain Sr 2 MnWO 6 Perovskite...
PUM
| Property | Measurement | Unit |
|---|---|---|
| half wave potential | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com