Structurally modified antibacterial peptide BMAP-14 and application thereof
A technology of BMAP-18 and antimicrobial peptide, applied in the field of protein engineering, can solve the problem of low hemolysis rate, achieve low hemolysis rate, retain broad-spectrum antibacterial properties, and good heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1: Construction of BMAP-14
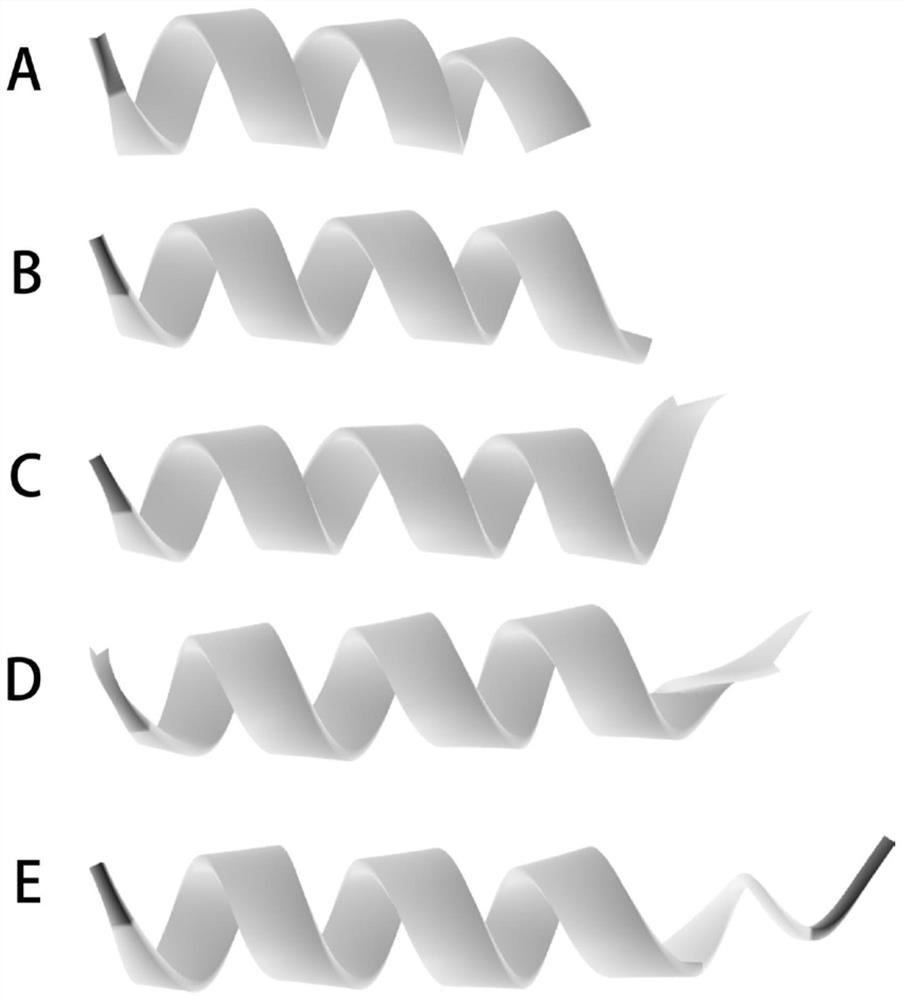
[0019] 1. Use I-TASSER to predict and establish the spatial structure of the unmodified antimicrobial peptide BMAP-18; design BMAP-14 with 4 amino acids reduced at the C-terminus of BMAP-18, and its amino acid sequence is shown in SEQ ID NO.1; At the same time, the C-terminus of BMAP-18 was designed to reduce 5, 3, and 2 polypeptides, respectively named as BMAP-13, BMAP-15, and BMAP-16. According to I-TASSER prediction, the spatial structure of each designed polypeptide is as follows: figure 1 shown. figure 1 Among them, from A to B, respectively correspond to: BMAP-13, BMAP-14, BMAP-15, BMAP-16, BMAP-18.
[0020] From figure 1 It can be seen that the BMAP-14 provided by the present invention is closest to the N-terminus of BMAP-18, and basically keeps the N-terminus of BMAP-18 unchanged, suggesting that it may maintain the antibacterial activity of the parent antimicrobial peptide BMAP-18; The spatial structures of the polypepti...
Embodiment 2
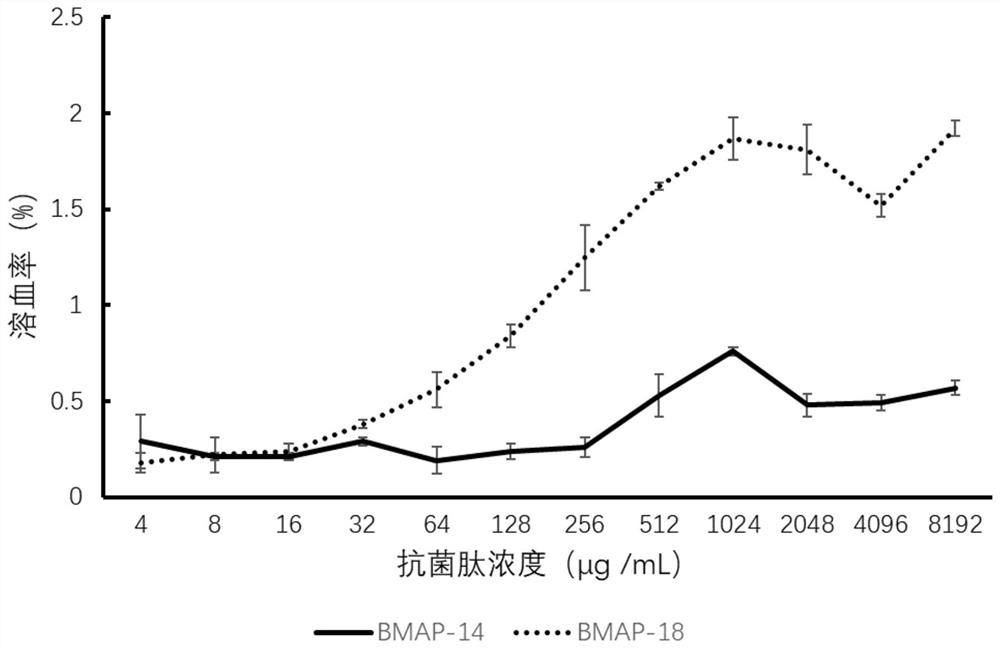
[0022] Embodiment 2: BMAP-14 performance detection
[0023] 1. Detection of antibacterial activity.
[0024] (1) Using the broth microdilution antibacterial test (M7-A8) of the American Clinical Laboratory Standardization Institute (CLSI), the minimum inhibitory concentration (MIC value) of BMAP-14 and BMAP-18 to bacteria was determined, and the culture The base is calcium-adjusted MH broth medium.
[0025] (2) The minimum inhibitory concentration of BMAP-14 and BMAP-18 against Candida albicans was determined by using the broth microdilution method anti-yeast-like fungal susceptibility test (M27-A2) of the American Clinical Laboratory Standardization Institute (CLSI) (MIC value), the detection medium is Sabouraud glucose liquid medium.
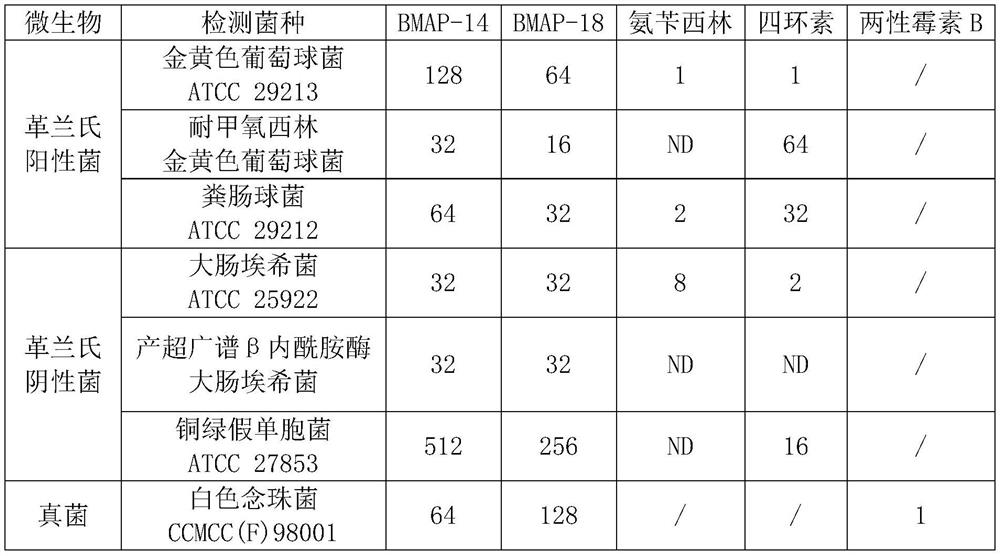
[0026] (3) The detection results are shown in Table 1. In Table 1, "ND" means that no activity was detected, and " / " means that no corresponding test was performed.
[0027] Table 1 The MIC value (μg / mL) of each antimicrobial peptide and c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com