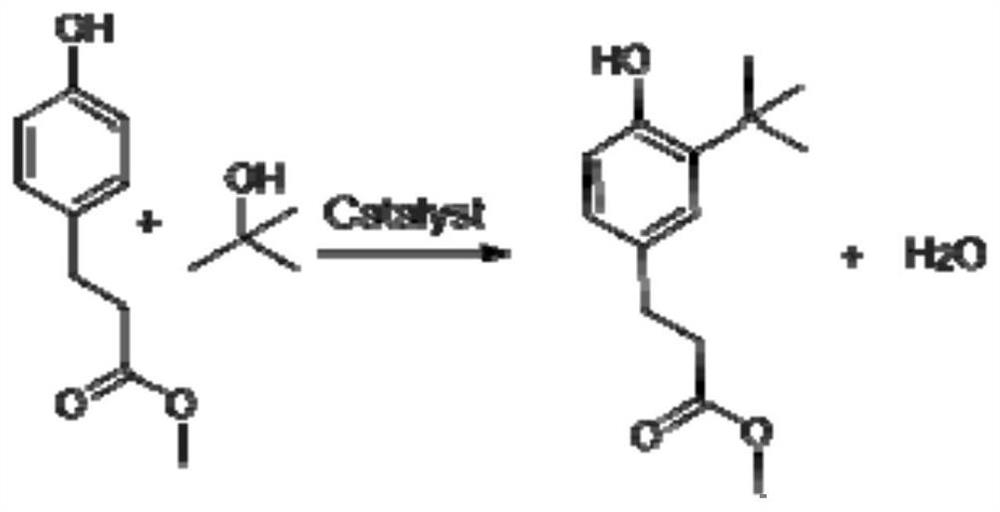
Synthesis process of methyl 3-(3-tert-butyl-4-hydroxy) phenylpropionate
A technology of methyl hydroxyphenylpropionate and methyl phenylpropionate, which is applied in the field of synthesis of methyl 3-phenylpropionate, can solve the problems of non-reusable catalysts, increased economic costs, and many by-products, and achieves The effect of good industrial production value, excellent quality and high utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
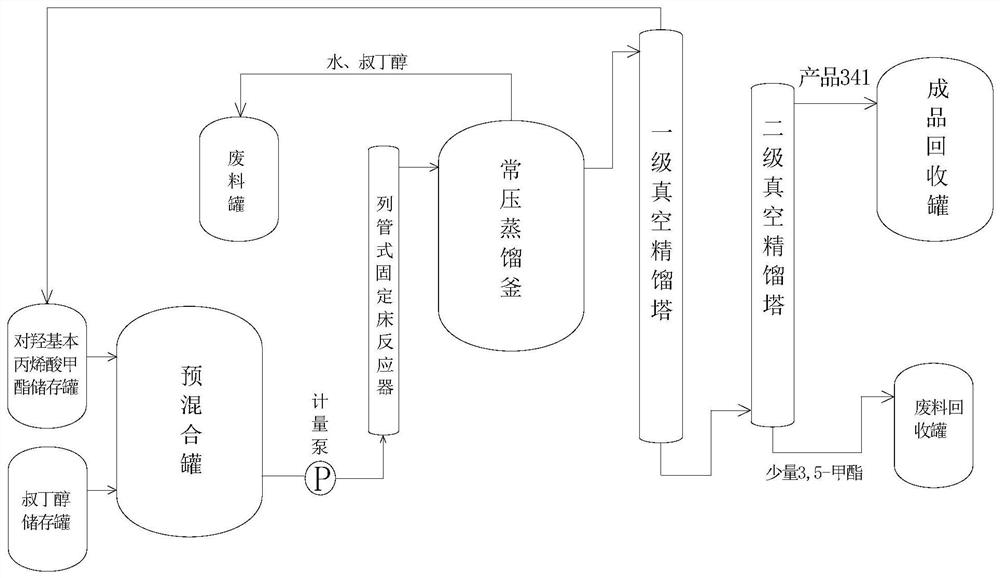
[0024] (1) Preparation of 3-(3-tert-butyl-4-hydroxyl) methyl phenylpropionate: 200 g of methyl p-hydroxyphenyl propionate and 58 g of tert-butanol (molar ratio is 1:0.7) are added to a temperature of Mix evenly in a pre-mixing tank at 40-45°C, and continuously add it to a column-and-tube fixed-bed reactor filled with granular solid sulfonic acid resin at a temperature of 100°C, an inner diameter of 2cm, and a packing height of 100cm through an advection pump with a flow rate of 2ml / min. The reaction is carried out in the tubular fixed bed reactor, and the average residence time of the material in the tubular fixed bed reactor is 1.04h. The thick product of methyl p-hydroxyphenylpropionate; sample is taken at the outlet of the reactor, and quantitatively analyzed by gas chromatography, the conversion rate of methyl p-hydroxyphenylpropionate is 63.31%, the conversion rate of tert-butyl alcohol is 91.02%, and the by-product 3-(35-di-tert-butyl The content of methyl-4-hydroxy)phen...
Embodiment 2
[0027] (1) Preparation of 3-(3-tert-butyl-4-hydroxyl) methyl phenylpropionate: 200 g of methyl p-hydroxyphenyl propionate and 58 g of tert-butanol (molar ratio is 1:0.7) are added to a temperature of Mix evenly in a pre-mixing tank at 40-45°C, and continuously add it to the column-and-tube fixed-bed reaction of packing granular solid sulfonic acid resin at a temperature of 100°C, an inner diameter of 2cm, and a packing height of 100cm through an advection pump with a flow rate of 1.5ml / min. The reaction is carried out in the reactor, and the average residence time of the material in the tubular fixed-bed reactor is 1.39h, and flows out from the outlet of the tubular fixed-bed reactor to obtain 3-(3-tert-butyl-4-hydroxyl)benzene The crude product of methyl propionate; sample is taken at the outlet of the reactor, and quantitatively analyzed by gas chromatography, the conversion rate of methyl p-hydroxyphenylpropionate is 63.97%, the conversion rate of tert-butyl alcohol is 92.13...
Embodiment 3
[0030](1) Preparation of 3-(3-tert-butyl-4-hydroxyl) methyl phenylpropionate: 200 g of methyl p-hydroxyphenyl propionate and 58 g of tert-butanol (molar ratio is 1:0.7) are added to a temperature of Mix evenly in a pre-mixing tank at 40-45°C, and continuously add it to a column-and-tube fixed-bed reactor filled with granular solid sulfonic acid resin at a temperature of 100°C, an inner diameter of 2cm, and a packing height of 100cm through an advection pump with a flow rate of 1ml / min. The reaction is carried out in the tubular fixed-bed reactor, and the average residence time of the material in the tubular fixed-bed reactor is 2.08h, and flows out from the outlet of the tubular fixed-bed reactor to obtain 3-(3-tert-butyl-4-hydroxyl) phenylpropane The crude product of methyl p-hydroxyphenyl propionate; sample is taken at the outlet of the reactor, and quantitatively analyzed by gas chromatography, the conversion rate of methyl p-hydroxyphenylpropionate is 63.97%, the conversion...
PUM
| Property | Measurement | Unit |
|---|---|---|
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com