Preparation process of novel PIN1 inhibitor
A technology of PIN1 and preparation process is applied in the field of preparation of novel PIN1 inhibitors to achieve the effects of reducing preparation cost, simple operation and less investment in equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
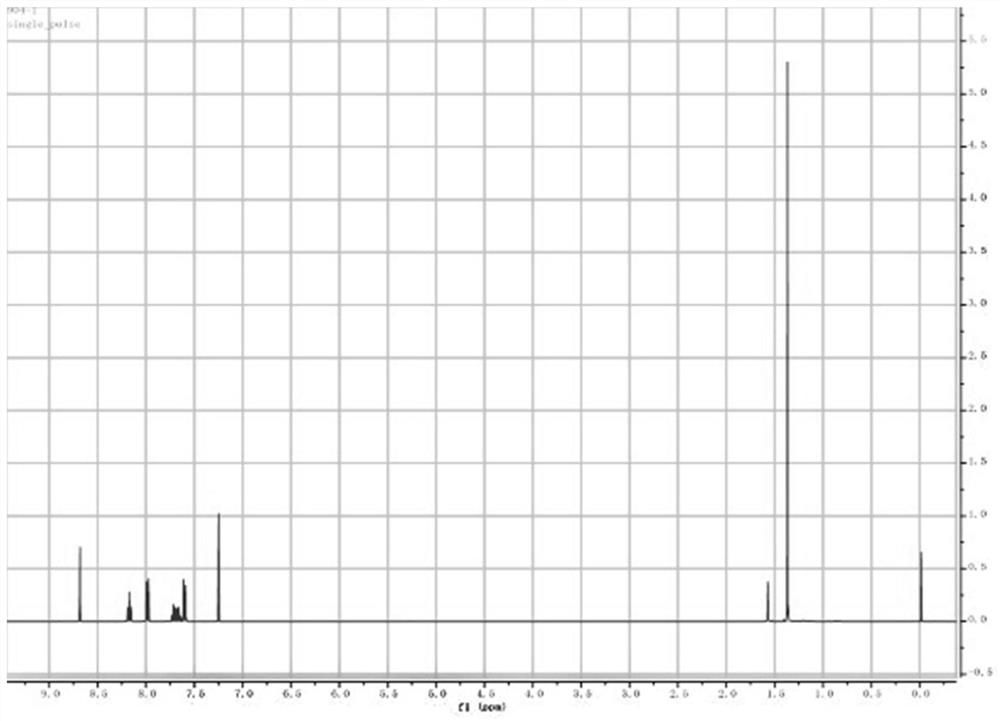
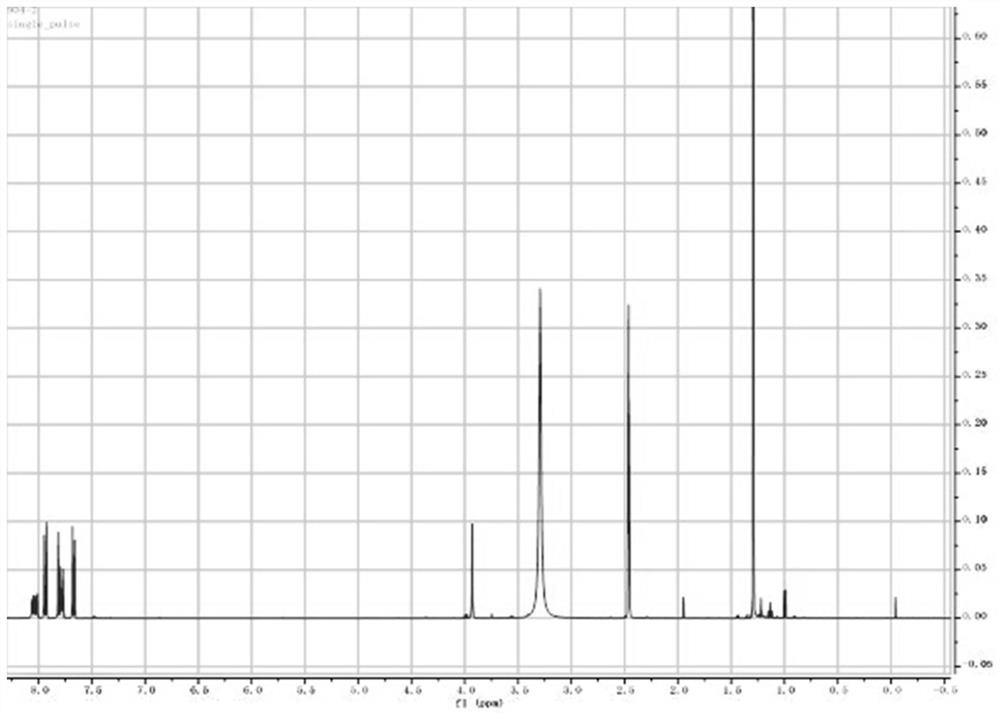
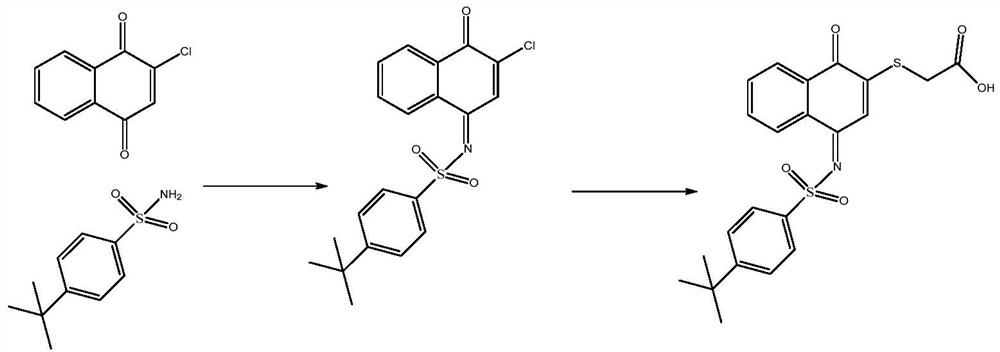
[0024] In a 350ml sealed tube, add 200ml tetrahydrofuran, 9.8g 2-chloro-1,4 naphthoquinone, 13g 4-butylbenthiamine, 2.1eq triethylamine, 1.2eq titanium tetrachloride, react at 60°C for 15h, TLC Track the reaction, spin dry, add petroleum ether and isopropyl ether to make a slurry. After suction filtration, the solid is the intermediate. Dissolve the intermediate in 200ml tetrahydrofuran, add 11g thioglycolic acid, 1.2eq pyridine, and stir at room temperature for 2 hours to complete the reaction. Spin the reaction solution to dryness, add isopropyl ether and tertiary methyl ether for beating , the product 2-{[4-(4-tert-butylbenzenesulfonamido)-1-oxo-1,4-dihydronaphthalene-2-yl]sulfanyl}acetic acid can be obtained, two-step yield was 67.4%.
Embodiment 2
[0026] In a sealed 20ml tube, add 10ml tetrahydrofuran, 0.8g 2-chloro-1,4-naphthoquinone, 1.1g 4-butylbenthiamine, 2.1eq triethylamine, 1.2eq titanium tetrachloride, and react at 60°C for 15h. TLC traced the reaction, spin-dried, added petroleum ether and isopropyl ether to make a slurry. After suction filtration, dissolve the solid in 200ml tetrahydrofuran, add 0.8g thioglycolic acid, 1.2eq pyridine, and stir at room temperature for 2 hours to complete the reaction. The reaction solution is spin-dried, and isopropyl ether and tertiary methyl ether are added to make a slurry to obtain the product 2- {[4-
[0027] (4-tert-butylbenzenesulfonamido)-1-oxo-1,4-dihydronaphthalen-2-yl]sulfanyl}acetic acid, the two-step yield was 57.4%.
Embodiment 3
[0029] In a sealed 40ml tube, add 30ml tetrahydrofuran, 1.8g 2-chloro-1,4 naphthoquinone, 2.1g 4-butylbenthiamine, 2.1eq triethylamine, 1.2eq titanium tetrachloride, react at 60°C for 15h, TLC traced the reaction, spin-dried, added petroleum ether and isopropyl ether to make a slurry. After suction filtration, the solid was dissolved in 200ml of tetrahydrofuran, 1.8g of thioglycolic acid and 1.2eq of pyridine were added, and the reaction was completed after stirring at room temperature for 2 hours. {[4-
[0030] (4-tert-butylbenzenesulfonamido)-1-oxo-1,4-dihydronaphthalen-2-yl]sulfanyl}acetic acid, the two-step yield was 65.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com